Abstract
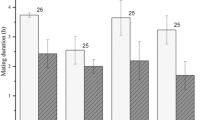
In many species the high energetic demands of reproduction induce a negative energy balance, and thus females must rely on tissue catabolism to complete the reproductive process. Previous works have shown that both fat and protein are energy resources during prolonged fasting in vertebrates. While many ecological studies on energy costs of reproduction have focused on variations in fat stores, the impact of protein investment on the female has not been thoroughly investigated. Notably, as there is no specialized storage form for proteins, intense catabolism is likely to entail structural (musculature) loss that may compromise maternal physical performance after reproduction. Measurements on captive rainbow boas (Epicrates cenchria maurus) confirm that reproducing females undergo significant protein catabolism (as indicated by elevated plasma uric acid levels) and show considerable musculature loss during gestation (as detected by reduced width of the epaxial muscles). Protein mobilization entailed a significant functional loss that was illustrated by decrements in tests of strength and constriction after parturition. In wild situations, such effects are likely to decrease the snakes’ ability to forage and apprehend prey. Hence, the time period needed to recover from reproduction can be extended not only because the female must compensate losses of both fat stores and functional muscle, but also because the ability to do so may be compromised. Performance alteration is likely to be of equal or greater importance than reduced energy stores in the physiological mediation of elevated post-reproduction mortality rates and infrequent reproductive bouts (e.g. biannual or triannual), two common ecological traits of female snakes.





Similar content being viewed by others
References
Birchard GF, Black CP, Schuett GW, Black V (1984) Influence of pregnancy on oxygen consumption, heart rate and haematology in the garter snake: implications for the cost of reproduction in live bearing reptiles. Comp Bioch Physiol 77:519–523
Bonnet X (1996) Gestion des réserves corporelles et stratégie de reproduction chez Vipera aspis. Thèse de Doctorat, Université Claude Bernard, Lyon I
Bonnet X, Naulleau G (1994) Utilisation d’un indice de condition corporelle (BCI) pour l’étude de la reproduction chez les serpents. C R Acad Sci 317:34–41
Bonnet X, Bradshaw SD, Shine R (1998) Capital versus income breeding: an ectothemic perspective. Oikos 83:333–341
Bonnet X, Naulleau G, Shine R, Lourdais O (1999) What is the appropriate time scale for measuring costs of reproduction in a capital breeder? Evol Ecol 13:485–497
Bonnet X, Naulleau G, Shine R, Lourdais O (2001) Short-term versus long-term effects of food intake on reproductive output in a viviparous snake (Vipera aspis). Oikos 92:297–308
Bonnet X, Lourdais O, Shine R, Naulleau G (2002a) Reproduction in snakes (Vipera aspis): costs, currencies and complications. Ecology 83:2124–2135
Bonnet X, Naulleau G, Lourdais O (2002b) The benefits of complementary techniques: using capture-recapture and physiological approaches to understand costs of reproduction in the asp viper. In: Schuett GW, Hoggren M, Greene HW (eds) Biology of the vipers. Biological Science Press, Traverse City, MI
Bordel R, Haase E (2000) Influence of flight on protein catabolism, especially myofilament breakdown, in homing pigeons. J Comp Physiol B 170:51–58
Brown WS (1993) Biology, status and management of the timber rattlesnake (Crotalus horridus): a guide for conservation. Herpetol Circ 22:1-78
Bull JJ, Shine R (1979) Iteroparous animals that skip opportunities for reproduction. Am Nat 114:296–316
Calow P (1979) The cost of reproduction—a physiological approach. Biol Rev 54:23–40
Charland MB, Gregory PT (1990) The influence of female reproductive status on thermoregulation in a viviparous snakes, Crotalus viridis. Copeia 1990:1089–1098
Chastel O, Weimerskirch H, Jouventin P (1995) The influence of body condition on reproductive decisions and reproductive success in the blue petrel. Ecology 76:2240–2246
Cherel Y, Grocolas R (1999) Relationship between nutrient storage and nutrient utilization in long-term fasting birds and mammals. In: Adams NJ, Slotow RH (eds) Proceedings of the 22nd International Ornithology Congress. Durbam, Johannesburg, pp 17–34
Cherel Y, Robin JP, Walch O, Karmann H, Netchitailo P, Le Maho Y (1988) Fasting in king penguin. 1. Hormonal and metabolic changes during breeding. Am J Physiol 254:R170–R177
Cherel Y, Robin JP, Heitz A, Calgari C, Le Maho Y (1992) Relationships between lipid availability and protein utilization during prolonged fasting. J Comp Physiol B 162:305–313
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton, New Jersey
Crocker DE, Webb PM, Costa DP, Le Boeuf BJ (1998) Protein catabolism and renal function in lactating northern elephant seals. Physiol Zool 71:485–491
Crocker DE, Williams JD, Costa DP and Le Boeuf BJ (2001) Maternal traits and reproductive effort in northern elephant seals Ecology 82:3541–3555
Cundall (1987) Functional morphology. In: Seigel RA, Collins JT, Novak SS (eds) Snakes: ecology and evolutionary biology. Macmillan, New York, pp 210–252
Doughty P, Shine R (1997) Detecting life history trade-offs: measuring energy stores in “capital” breeders reveals costs of reproduction. Oecologia 110:508–513
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 68:225–252
Goodman MN, Lowell B, Belur E, Ruderman NB (1984) Sites of protein conservation and loss during starvation- Influence of adiposity. Am J Physiol 246:E383–E390
Gregory PT, Skebo KM (1998) Trade-offs between reproductive traits and the influence of food intake during pregnancy in the garter snake, Thamnophis elegans. Am Nat 151:477–486
Gregory PT, Lisa HC, Skebo KM (1999) Conflicts and interactions among reproduction, thermoregulation and feeding in viviparous reptiles: are gravid snakes anorexic? J Zool (London) 248:231–241
Guillette LJ (1982) Effects of gravidity on the metabolism of the reproductively bimodal lizard, Sceloporua avenues. J Exp Zool 223:33–36
Henen BT (1997) Seasonal and annual energy budget of female desert tortoise (Gopherus agassizii). Ecology 78:283–296
Henen BT (2002) Reproductive effort and reproductive nutrition of female desert tortoises: essential field methods. Integr Comp Biol 42:43–50
Houston DC, Donnan D, Jones PJ (1995) Use of labeled methionine to investigate the contribution of muscle proteins to egg production in zebra finches. J Comp Physiol B 165:161–164
Huey BR (1982) Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough HF (eds) Biology of the Reptilia, vol 12. Physiological ecology. Academic Press, pp 25–95
Jönsson KI (1997) Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78:57–66
Jönsson KI, Tuomi J, Järemo J (1995) Reproductive effort tactics: balancing pre- and postbreeding costs of reproduction. Oikos 74:35–44
Ladyman M, Bonnet X, Lourdais O, Bradshaw D, Naulleau G (2003) Gestation, thermoregulation and metabolism in a viviparous snake, Vipera aspis: evidence for fecundity-independent costs. Physiol Biochem Zool 76:497–510
Lourdais O, Bonnet X, Doughty P (2002a) Costs of anorexia during pregnancy in a viviparous snake (Vipera aspis). J Exp Zool 292:487–493
Lourdais O, Bonnet X, Shine R, DeNardo D, Naulleau G, Guillon M (2002b) Capital-breeding and reproductive effort in a variable environment: a longitudinal study of a viviparous snake. J Anim Ecol 71:470–479
Lowell BB, Goodman MN (1987) Protein sparing in skeletal-muscle during prolonged starvation—dependence on lipid fuel availability. Diabetes 36:14–19
Lowell BB, Ruderman NB, Goodman MN (1986) Regulation of myofibrillar protein degradation in rat skeletal muscle during brief and prolonged starvation. Metabolism 35:1121–1127
Madsen T, Shine R (1993) Costs of reproduction in a population of European adders. Oecologia 94:488–495
Madsen T, Shine R (1999a) The adjustment of reproductive threshold to prey abundance in a capital breeder. J Anim Ecol 68:571–580
Madsen T, Shine R (1999b) Life history consequences of nest-site variation in tropical pythons (Liasis fuscus). Ecology 80:989–997
Matz G (2001) Biologie de la reproduction de Epicrates maurus GRAY, 1849. Situla 3:10–15
Meijer T, Drent R (1999) Re-examination of the capital and income dichotomy in breeding birds. Ibis 141:399–414
Moon BR (2000) The mechanics and muscular control of constriction in gopher snakes (Pituophis melanoleucus) and a king snake (Lampropeltis getula). J Zool (London) 252:83–98
Oftedal OT (1993) The adaptation of milk secretion to the constraints of fasting in bears, seals, and baleen whales. J Dairy Sci 76:3234–3246
Packard GC, Packard MJ (1988) The physiological ecology of reptilian eggs and embryos. In: Gans C, Huey R (eds) Biology of the Reptilia. Liss, New York, pp 523–605
Pearson D, Shine R, Williams A (2003) Thermal biology of large snakes in cool climates: a radio-telemetric study of carpet pythons (Morelia spilota imbricata) in south-western Australia. J Therm Biol 28:117–131
Rivas JA (1999) Life history of the green anaconda (Eunectes murinus) with emphasis on its reproductive biology. Ph.D. thesis, University of Tennessee, Knoxville
Ross RA, Marzec G (1990) The reproductive husbandry of pythons and boas. institute for herpetological research. Standford, California
Schwilch R, Grattarola A, Spina F, Jenni L (2002) Protein loss during long-distance migratory flight in passerine birds: adaptation and constraint. J Exp Biol 205:687–695
Sheridan MA (1994) Regulation of lipid-metabolism in poikilothermic vertebrates. C Biochem Physiol B 107:495–508
Shine R (1980) “Costs” of reproduction in reptiles. Oecologia 46:92–100
Shine R (1985) The evolution of viviparity in reptiles: an ecological analysis. In: Gans C, Billet F (eds) Biology of the Reptilia, vol. 15. Wiley, New York, pp 605–694
Shine R (1991) Influences of incubation requirements on the evolution of viviparity. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 361–370
Slip DJ, Shine R (1988) Reptilian endothermy: a field study of thermoregulation by brooding diamond pythons. J Zool (London) 216:367–378
Somma LA (2003) Parental behavior in lepidosaurian and testudinian reptiles, a literature survey. Krieger, Malabar, Florida
White HB (1991) Maternal diet, maternal proteins and egg quality. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 1–15
Acknowledgements
We thank Gwenaël Beauplet and Xavier Bonnet for helpful comments on the manuscript. We are grateful to Gilbert Matz for providing the snake colony. Financial support was provided by the Conseil Général des Deux-Sèvres, the CNRS, and the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: I.D. Hume
Rights and permissions
About this article
Cite this article
Lourdais, O., Brischoux, F., DeNardo, D. et al. Protein catabolism in pregnant snakes (Epicrates cenchria maurus Boidae) compromises musculature and performance after reproduction. J Comp Physiol B 174, 383–391 (2004). https://doi.org/10.1007/s00360-004-0424-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-004-0424-6