Abstract
Purpose
There are no definitive prognostic factors for patients with pathological Grade Group 5 (pGG 5) prostate cancer (PCa) undergoing robot-associated radical prostatectomy (RARP). This study aimed to explore the prognostic factors among patients with pGG 5 PCa in a large Japanese cohort (MSUG94).
Methods
This retrospective, multi-institutional cohort study was conducted between 2012 and 2021 at ten centers in Japan and included 3195 patients. Patients with clinically metastatic PCa (cN1 or cM1) and those receiving neoadjuvant and/or adjuvant therapy were excluded. Finally, 217 patients with pGG5 PCa were analyzed.
Results
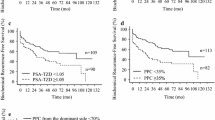
The median follow-up period was 28.0 months. The 3- and 5-year biochemical recurrence-free survival (BCRFS) rates of the overall population were 66.1% and 57.7%, respectively. The optimal threshold value (47.2%) for the percentage of positive cancer cores (PPCC) with any GG by systematic biopsy was chosen based on receiver operating characteristic curve analysis. Univariate analysis revealed that the prostate-specific antigen level at diagnosis, pT, pN, positive surgical margins (PSMs), lymphovascular invasion, and PPCC were independent prognostic factors for BCRFS. A multivariate analysis revealed that PSMs and PPCC were independent prognostic factors for BCRFS. Using these two predictors, we stratified BCRFS, metastasis-free survival (MFS), and castration-resistant PCa-free survival (CRPC-FS) among patients with pGG 5 PCa.
Conclusion
The combination of PSMs and PPCC may be an important predictor of BCRFS, MFS, and CRPC-FS in patients with pGG 5 PCa undergoing RARP.


Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Ciezki JP, Weller M, Reddy CA, Kittel J, Singh H, Tendulkar R, Stephans KL, Ulchaker J, Angermeier K, Stephenson A et al (2017) A comparison between low-dose-rate brachytherapy with or without androgen deprivation, external beam radiation therapy with or without androgen deprivation, and radical prostatectomy with or without adjuvant or salvage radiation therapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 97:962–975. https://doi.org/10.1016/j.ijrobp.2016.12.014
Campbell JM, Raymond E, O’Callaghan ME, Vincent AD, Beckmann KR, Roder D, Evans S, McNeil J, Millar J, Zalcberg J et al (2017) Optimum tools for predicting clinical outcomes in prostate cancer patients undergoing radical prostatectomy: a systematic review of prognostic accuracy and validity. Clin Genitourin Cancer 15:e827–e834. https://doi.org/10.1016/j.clgc.2017.06.001
Garg H, Dursun F, Alsayegh F, Wang H, Wu S, Liss MA, Kaushik D, Svatek RS, Mansour AM (2023) Revisiting current National Comprehensive Cancer Network (NCCN) high-risk prostate cancer stratification: a National Cancer Database analysis. Prostate Cancer Prostatic Dis 14:10. https://doi.org/10.1038/s41391-022-00621-7
Wilkins LJ, Tosoian JJ, Reichard CA, Sundi D, Ranasinghe W, Alam R, Schwen Z, Reddy C, Allaf M, Davis JW et al (2021) Oncologic outcomes among Black and White men with grade group 4 or 5 (Gleason score 8–10) prostate cancer treated primarily by radical prostatectomy. Cancer 127:1425–1431. https://doi.org/10.1002/cncr.33419
Panunzio A, Sorce G, Hoeh B, Hohenhorst L, Tappero S, Nimer N, Rajwa P, Tian Z, Terrone C, Chun FKH et al (2023) Effect of positive surgical margins at radical prostatectomy on cancer-specific mortality in high/very high-risk prostate cancer patients with Gleason Grade Group 4–5. Prostate 83:268–276. https://doi.org/10.1002/pros.24458
Kamitani R, Matsumoto K, Kosaka T, Takeda T, Hashiguchi A, Tanaka N, Morita S, Mizuno R, Shinojima T, Asanuma H, Oya M (2021) Evaluation of Gleason Grade Group 5 in a contemporary prostate cancer grading system and literature review. Clin Genitourin Cancer 19:69-75.e5e65. https://doi.org/10.1016/j.clgc.2020.08.001
Ellis CL, Partin AW, Han M, Epstein JI (2013) Adenocarcinoma of the prostate with Gleason score 9–10 on core biopsy: correlation with findings at radical prostatectomy and prognosis. J Urol 190:2068–2073. https://doi.org/10.1016/j.juro.2013.05.056
Wenzel M, Würnschimmel C, Chierigo F, Mori K, Tian Z, Terrone C, Shariat SF, Saad F, Tilki D, Graefen M et al (2022) Pattern of biopsy Gleason Grade Group 5 (4 + 5 vs 5 + 4 vs 5 + 5) predicts survival after radical prostatectomy or external beam radiation therapy. Eur Urol Focus 8:710–717. https://doi.org/10.1016/j.euf.2021.04.011
Lattouf JB, Saad F (2002) Gleason score on biopsy: is it reliable for predicting the final grade on pathology? BJU Int 90:694–698. https://doi.org/10.1046/j.1464-410x.2002.02990.x. (discussion 698–699)
Kato D, Ebara S, Tatenuma T, Sasaki T, Ikehata Y, Nakayama A, Toide M, Yoneda T, Sakaguchi K, Teishima J et al (2022) Short-term oncological and surgical outcomes of robot-assisted radical prostatectomy: a retrospective multicenter cohort study in Japan (the MSUG94 group). Asian J Endosc Surg 15:745–752. https://doi.org/10.1111/ases.13074
Sasaki T, Ebara S, Tatenuma T, Ikehata Y, Nakayama A, Kawase M, Toide M, Yoneda T, Sakaguchi K, Teishima J et al (2023) Prognostic differences among the positive surgical margin locations following robot-assisted radical prostatectomy in a large Japanese cohort (the MSUG94 group). Jpn J Clin Oncol 53:443–451. https://doi.org/10.1093/jjco/hyad004
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F et al (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65:467–479. https://doi.org/10.1016/j.eururo.2013.11.002
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Committee (2016) The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40:244–252. https://doi.org/10.1097/PAS.0000000000000530
Moschini M, Sharma V, Soligo M, Psutka S, Rangel L, Boorjian SA, Frank I, Gettman MT, Thompson RH, Tollefson MK, Karnes RJ (2018) Heterogeneity of risk within Gleason 4 + 4, 4 + 5 and 5 + 4 prostate cancer. Scand J Urol 52:340–348. https://doi.org/10.1080/21681805.2018.1534886
Tilki D, Würnschimmel C, Preisser F, Graefen M, Huland H, Mandel P, Tennstedt P (2020) The significance of primary biopsy Gleason 5 in patients with grade Group 5 prostate cancer. Eur Urol Focus 6:255–258. https://doi.org/10.1016/j.euf.2020.01.008
Kishan AU, Shaikh T, Wang PC, Reiter RE, Said J, Raghavan G, Nickols NG, Aronson WJ, Sadeghi A, Kamrava M et al (2017) Clinical outcomes for patients with Gleason score 9–10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: a multi-institutional comparative analysis. Eur Urol 71:766–773. https://doi.org/10.1016/j.eururo.2016.06.046
Kishan AU, Cook RR, Ciezki JP, Ross AE, Pomerantz MM, Nguyen PL, Shaikh T, Tran PT, Sandler KA, Stock RG et al (2018) Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with Gleason score 9–10 prostate cancer. JAMA 319:896–905. https://doi.org/10.1001/jama.2018.0587
Muralidhar V, Mahal BA, Butler S, Lamba N, Yang DD, Leeman J, D’Amico AV, Nguyen PL, Trinh QD, Orio PF 3rd, King MT (2019) Combined external beam radiation therapy and brachytherapy versus radical prostatectomy with adjuvant radiation therapy for Gleason 9–10 prostate cancer. J Urol 202:973–978. https://doi.org/10.1097/JU.0000000000000352
Knipper S, Palumbo C, Pecoraro A, Rosiello G, Tian Z, Briganti A, Zorn KC, Saad F, Tilki D, Graefen M, Karakiewicz PI (2020) Survival outcomes of radical prostatectomy vs. external beam radiation therapy in prostate cancer patients with Gleason Score 9–10 at biopsy: a population-based analysis. Urol Oncol 38:79.e9-79.e14. https://doi.org/10.1016/j.urolonc.2019.09.015
Chao HH, Soni PD, Dahman B, Stilianoudakis SC, Ford H, Singh R, Freedland SJ, Moghanaki D, Vapiwala N, Chang MG (2022) Outcomes following radical prostatectomy or external beam radiation for veterans with Gleason 9 and 10 prostate cancer. Cancer Med 11:2886–2895. https://doi.org/10.1002/cam4.4656
Flood TA, Schieda N, Sim J, Breau RH, Morash C, Belanger EC, Robertson SJ (2018) Evaluation of tumor morphologies and association with biochemical recurrence after radical prostatectomy in Grade Group 5 prostate cancer. Virchows Arch 472:205–212. https://doi.org/10.1007/s00428-017-2241-9
Velho PI, Lim D, Wang H, Park JC, Kaur HB, Almutairi F, Carducci MA, Denmeade SR, Markowski MC, Isaacs WB et al (2019) Molecular characterization and clinical outcomes of primary Gleason Pattern 5 prostate cancer after radical prostatectomy. JCO Precis Oncol. https://doi.org/10.1200/PO.19.00081
Antunes AA, Srougi M, Dall’Oglio MF, Crippa A, Campagnari JC, Leite KR (2005) The percentage of positive biopsy cores as a predictor of disease recurrence in patients with prostate cancer treated with radical prostatectomy. BJU Int 96:1258–1263. https://doi.org/10.1111/j.1464-410X.2005.05823.x
Yamashita S, Kohjimoto Y, Sato H, Kikkawa K, Sonomura T, Hara I (2022) PI-RADS v2 findings of MRI and positive biopsy core percentage would predict pathological extraprostatic extension in patients who underwent robot assisted radical prostatectomy: a retrospective study. Urol J 19:438–444. https://doi.org/10.22037/uj.v19i.6923
Gandaglia G, Ploussard G, Valerio M, Mattei A, Fiori C, Fossati N, Stabile A, Beauval JB, Malavaud B, Roumiguié M et al (2019) A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur Urol 75:506–514. https://doi.org/10.1016/j.eururo.2018.10.012
Van den Broeck T, van den Bergh RCN, Arfi N, Gross T, Moris L, Briers E, Cumberbatch M, De Santis M, Tilki D, Fanti S et al (2019) Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur Urol 75:967–987. https://doi.org/10.1016/j.eururo.2018.10.011
Acknowledgements
We would like to thank Editage (https://www.editage.com) for English language editing.
Funding
This study received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Takeshi Sasaki: Data collection and management, data analysis, and manuscript writing/editing. Shin Ebara: Protocol/project development and data collection and management. Tomoyuki Tatenuma: Data collection and management. Yoshinori Ikehata: Data collection and management. Akinori Nakayama: Data collection and management. Makoto Kawase: Protocol/project development and data collection and management. Masahiro Toide: Data collection and management. Tatsuaki Yoneda: Protocol/project development and data collection and management. Kazushige Sakaguchi: Data collection and management. Jun Teishima: Protocol/project development and supervision. Kazuhide Makiyama: Protocol/project development and supervision. Hiroshi Kitamura: Protocol/project development and supervision. Kazutaka Saito: Protocol/project development and supervision. Takuya Koie: Protocol/project development and supervision. Fumitaka Koga: Protocol/project development and supervision. Shinji Urakami: Protocol/project development and supervision. Takahiro Inoue: Protocol/project development, data management, and manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Approval of the research protocol by an Institutional Reviewer Board
The protocol for this research project was approved by the suitably constituted ethics committee of each institution and conducted in accordance with the provisions of the Declaration of Helsinki (Mie University Hospital Clinical Research Ethics Committee, approval no. H2021-175).
Informed consent
The requirement for informed consent was waived because the study was retrospective and observational.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
345_2024_4864_MOESM1_ESM.pdf
Supplementary file1 Supplemental Figure 1. A Kaplan–Meier analysis of BCRFS, MFS, and CRPC-FS in patients with pGG 5 stratified by PSM location based on RARP specimens. A. The 3-year BCRFS rates were 52.9%, 37.2%, 31.6%, 60.0%, and 55.6% in patients with apex-only, middle-only, bladder neck-only, seminal vesicle-only, and multifocal PSMs, respectively (P = 0.69). B. The 3-year MFS rates were 95.8%, 78.8%, 95.0%, 80.0%, and 100% in patients with apex-only, middle-only, bladder neck-only, seminal vesicle-only, and multifocal PSMs, respectively (P = 0.38). C. The 3-year CRPC-FS rates were 94.7%, 88.9%, 84.4%, 100%, and 100% in patients with apex-only, middle-only, bladder neck-only, seminal vesicle-only, and multifocal PSMs, respectively (P = 0.81). BCRFS, biochemical recurrence-free survival; MFS, metastasis-free survival; CRPC-FS, castration-resistant prostate cancer-free survival; GG, Grade Group; PSM, positive surgical margin; RARP, robot-associated radical prostatectomy (PDF 1440 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sasaki, T., Ebara, S., Tatenuma, T. et al. Prognostic factors among patients with pathological Grade Group 5 prostate cancer based on robot-associated radical prostatectomy specimens from a large Japanese cohort (MSUG94). World J Urol 42, 152 (2024). https://doi.org/10.1007/s00345-024-04864-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04864-y