Abstract
Purpose
Oxalate is an excellent calcium ion attractor with great abundance in the human body, and the liver is the major source of oxalate. The Glycolate oxidase-1 (GOX1) gene is solely responsible for the glycolate and glyoxylate metabolism and produces oxalate. This study has been designed to comprehend the association of genetic variants of the GOX1 gene with the risk of hyperoxaluria and renal stone disease in the Indian population.
Method
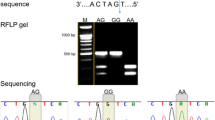
The present study is a candidate gene approach prospective case–control study carried out on 300 participants (150 cases and 150 controls) at Muljibhai Patel Urological Hospital, Gujarat, India. Biochemical parameters, including serum levels of calcium, creatinine, parathyroid hormone, and 24-h urine metabolites, were performed. The genotyping of GOX1 gene variants rs6086287, rs2235250, rs2255183, and rs2294303 was performed using a customized TaqMan assay probe by RT-PCR.
Result
Parathyroid hormone, serum creatinine, and urine metabolites were significantly elevated in nephrolithiasis compared to healthy individuals. All mutated homozygous genotypes GG (rs6086287), TT (rs2235250), GG (rs2255183), and CC (rs2294303) were significantly associated with a high risk of renal stone disease. Individuals diagnosed with hyperoxaluria and carrying TG (rs6086287), AG (rs2255183), and TT (rs2294303) genotypes have a significantly high risk of renal stone disease. Moreover, haplotype analysis and correlation analysis also confirmed the strong association between genetic variants and nephrolithiasis.
Conclusion
Genetic variants of the GOX1 genes were associated with renal stone disease. In the presence of risk genotype and hyperoxaluria, the susceptibility to develop renal stone disease risk gets modulated.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alelign T, Petros B (2018) Kidney stone disease: an update on current concepts. Adv Urol 2018:3068365. https://doi.org/10.1155/2018/3068365
Sigurjonsdottir VK, Runolfsdottir HL, Indridason OS et al (2015) Impact of nephrolithiasis on kidney function. BMC Nephrol 16:1–7. https://doi.org/10.1186/S12882-015-0126-1/TABLES/3
Rule AD, Roger VL, Melton LJ et al (2010) Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21:1641–1644. https://doi.org/10.1681/ASN.2010030253
Taylor EN, Stampfer MJ, Curhan GC (2005) Obesity, weight gain, and the risk of kidney stones. JAMA 293:455–462. https://doi.org/10.1001/JAMA.293.4.455
Alelign T, Petros B (2018) Kidney stone disease: an update on current concepts. Adv Urol. https://doi.org/10.1155/2018/3068365
Narula S, Tandon S, Kumar D et al (2020) Human kidney stone matrix proteins alleviate hyperoxaluria induced renal stress by targeting cell-crystal interactions. Life Sci 262:118498. https://doi.org/10.1016/J.LFS.2020.118498
Huang Y, Zhang YH, Chi ZP et al (2020) The handling of oxalate in the body and the origin of oxalate in calcium oxalate stones. Urol Int 104:167–176. https://doi.org/10.1159/000504417
McGregor TL, Hunt KA, Yee E et al (2020) Characterising a healthy adult with a rare HAO1 knockout to support a therapeutic strategy for primary hyperoxaluria. Elife 9:e54363–e54363. https://doi.org/10.7554/ELIFE.54363
Jones JM, Morrell JC, Gould SJ (2000) Identification and characterization of HAOX1, HAOX2, and HAOX3, three human peroxisomal 2-hydroxy acid oxidases. J Biol Chem 275:12590–12597. https://doi.org/10.1074/jbc.275.17.12590
Bhasin B, Ürekli HM, Atta MG (2015) Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol 4:235. https://doi.org/10.5527/WJN.V4.I2.235
Rothe HM, Liangos O, Biggar P et al (2011) Cinacalcet treatment of primary hyperparathyroidism. Int J Endocrinol. https://doi.org/10.1155/2011/415719
Dsouza-Li L, Canaff L, Janicic N et al (2001) An acceptor splice site mutation in the calcium-sensing receptor (CASR) gene in familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Hum Mutat 18:411–421. https://doi.org/10.1002/HUMU.1212
Moe SM, Wetherill L, Decker BS et al (2017) Calcium-sensing receptor genotype and response to cinacalcet in patients undergoing hemodialysis. Clin J Am Soc Nephrol 12:1128–1138. https://doi.org/10.2215/CJN.11141016
Lila AR, Sarathi V, Jagtap V et al (2012) Renal manifestations of primary hyperparathyroidism. Indian J Endocrinol Metab 16:258. https://doi.org/10.4103/2230-8210.93745
Rejnmark L, Vestergaard P, Mosekilde L (2011) Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab 96:2377–2385. https://doi.org/10.1210/JC.2011-0569
Reid LJ, Muthukrishnan B, Patel D et al (2019) Predictors of nephrolithiasis, osteoporosis, and mortality in primary hyperparathyroidism. J Clin Endocrinol Metab 104:3692–3700. https://doi.org/10.1210/JC.2018-02483
Craven BL, Passman C, Assimos DG (2008) Hypercalcemic states associated with nephrolithiasis. Rev Urol 10:218
Renkema KY, Lee K, Topala CN et al (2009) TRPV5 gene polymorphisms in renal hypercalciuria. Nephrol Dial Transplant 24:1919–1924. https://doi.org/10.1093/NDT/GFN735
Pak CYC, Sakhaee K, Moe OW et al (2011) Defining hypercalciuria in nephrolithiasis. Kidney Int 80:777–782. https://doi.org/10.1038/KI.2011.227
Rungroj N, Nettuwakul C, Sudtachat N et al (2014) A whole genome SNP genotyping by DNA microarray and candidate gene association study for kidney stone disease. BMC Med Genet 15:1–11. https://doi.org/10.1186/1471-2350-15-50/TABLES/5
Acknowledgements
We want to thank the knowledge consortium of Gujarat (KCG), India, for providing scholarship and contingency. We are also thankful to Charotar University of Science and Technology (CHARUSAT) for facilitating us with CHARUSAT SEED RESEARCH GRANT(CHARUSATSEEDRESEARCHGRANT/RPCP/SAPA) and Muljibhai Patel Urological Hospital (MPUH) for providing financial assistance (Department of Urology Research Support Grant) to carried out research work.
Author information
Authors and Affiliations
Contributions
YP: conceptualization, data curation, investigation, resources, visualization, writing—original draft preparation. SBP: conceptualization, visualization, writing—review and editing. PP: investigation. AP: investigation. SS: methodology. RS: writing—review and editing. CR: formal analysis. AG: conceptualization, project administration, supervision, writing—review and editing. MRD: funding acquisition, supervision. SGP: conceptualization, project administration, resources, supervision, visualization, writing—review and editing. SNP: conceptualization, methodology, resources, software, supervision, validation, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human studies
The protocol for this research project has been approved by the Muljibhai Patel Society for Research in Nephro-Urology at Muljibhai Patel Urological Hospital, Nadiad, Gujarat, India, on 29th July 2019, and the approval number is “EC/575/2019 and EC/697/2020”.
Informed consent
All informed consent forms were signed and obtained from the participants.
Animal studies
Not applicable.
Clinical trials registry
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, Y.P., Patel, S.B., Patel, P. et al. Glycolate oxidase-1 gene variants influence the risk of hyperoxaluria and renal stone development. World J Urol 42, 28 (2024). https://doi.org/10.1007/s00345-023-04718-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-023-04718-z