Abstract
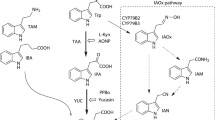
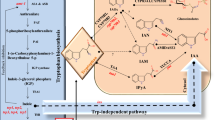
Although polar transport and the TIR1-dependent signaling pathway of the plant hormone auxin/indole-3-acetic acid (IAA) are well characterized, understanding of the biosynthetic pathway(s) leading to the production of IAA is still limited. Genetic dissection of IAA biosynthetic pathways has been complicated by the metabolic redundancy caused by the apparent existence of several parallel biosynthetic routes leading to IAA production. Valuable complementary tools for genetic as well as biochemical analysis of auxin biosynthesis would be molecular inhibitors capable of acting in vivo on specific or general components of the pathway(s), which unfortunately have been lacking. Several indole derivatives have been previously identified to inhibit tryptophan-dependent IAA biosynthesis in an in vitro system from maize endosperm. We examined the effect of one of them, 6-fluoroindole, on seedling development of Arabidopsis thaliana and tested its ability to inhibit IAA biosynthesis in feeding experiments in vivo. We demonstrated a correlation of severe developmental defects or growth retardation caused by 6-fluoroindole with significant downregulation of de novo synthesized IAA levels, derived from the stable isotope-labeled tryptophan pool, upon treatment. Hence, 6-fluoroindole shows important features of an inhibitor of tryptophan-dependent IAA biosynthesis both in vitro and in vivo and thus may find use as a promising molecular tool for the identification of novel components of the auxin biosynthetic pathway(s).




Similar content being viewed by others
References
Campanella JJ, Ludwig-Müller J, Bakllamaja V, Sharma V, Cartier A (2003) ILR1 and sILR1 IAA amidohydrolase homologs differ in expression pattern and substrate specificity. Plant Growth Regul 41:215–223
Chen K-H, Miller AN, Patterson GW, Cohen JD (1988) A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol 86:822–825
Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799
Cohen JD (1984) Convenient apparatus for the generation of small amounts of diazomethane. J Chromatogr 303:193–196
Cohen JD, Gray WM (2006) Auxin metabolism and signaling. In: Hedden P, Thomas S (eds) Plant hormone signaling (Annual Plant Reviews, Vol. 24). Blackwell Publishing, Oxford, pp 37–66
Delker C, Raschke A, Quint M (2008) Auxin dynamics: the dazzling complexity of a small molecule’s message. Planta 227:929–941
Ehlert B, Schöttler MA, Tischendorf G, Ludwig-Müller J, Bock R (2008) The paramutated SULFUREA locus of tomato is involved in auxin biosynthesis. J Exp Bot 59:3635–3647
Epstein E, Cohen JD, Slovin JP (2002) The biosynthetic pathway for indole-3-acetic acid changes during tomato fruit development. Plant Growth Regul 38:15–20
Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H (2008) Small-molecule agonists and antagonists of F-box protein–substrate interactions in auxin perception and signalling. Proc Natl Acad Sci USA 105:5632–5637
Ilic N, Östin A, Cohen JD (1999) Differential inhibition of indole-3-acetic acid and trpytophan biosynthesis by indole analogues. I. Tryptophan dependent IAA biosynthesis. Plant Growth Regul 27:57–62
Jentschel K, Thiel D, Rehn F, Ludwig-Müller J (2007) Arbuscular mycorrhiza enhances auxin levels and alters auxin biosynthesis in Tropaeolum majus during early stages of colonization. Physiol Plant 129:320–333
Keitt GW, Baker RA (1966) Auxin activity of substituted benzoic acids and their effect on polar auxin transport. Plant Physiol 41:1561–1569
Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2:1071–1080
Ludwig-Müller J, Sass S, Sutter EG, Wodner M, Epstein E (1993) Indole-3-butyric acid in Arabidopsis thaliana. I. Identification and quantification. Plant Growth Regul 13:179–187
Michalczuk L, Cooke TJ, Cohen JD (1992) Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry 31:1097–1103
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Normanly J, Cohen JD, Fink GR (1993) Arabidopsis thaliana auxotrophs reveal a tryptophan independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci USA 90:10355–10374
Normanly J, Slovin JP, Cohen JD (1995) Rethinking auxin biosynthesis and metabolism. Plant Physiol 107:323–329
O’Hagan D (2008) Understanding organofluorine chemistry. An introduction to the C–F bond. Chem Soc Rev 37:308–319
Östin A, Ilic N, Cohen JD (1999) An in vitro system from maize seedlings for tryptophan independent IAA biosynthesis. Plant Physiol 119:173–178
Piotrowski M (2008) Primary or secondary? Versatile nitrilases in plant metabolism. Phytochemistry 69:2655–2667
Pollmann S, Neu D, Weiler EW (2003) Molecular cloning and characterization of an amidase from Arabidopsis thaliana capable of converting indole-3-acetamide into the plant growth hormone, indole-3-acetic acid. Phytochemistry 62:293–300
Pollmann S, Müller A, Weiler EW (2006) Many roads lead to “auxin”: of nitrilases, synthases, and amidases. Plant Biol 8:326–333
Pollmann S, Düchting P, Weiler EW (2009) Tryptophan-dependent indole-3-acetic acid biosynthesis by ‘IAA-synthase’ proceeds via indole-3-acetamide. Phytochemistry 70:523–531
Quint M, Gray WM (2006) Auxin signaling. Curr Opin Plant Biol 9:448–453
Quint M, Barkawi LS, Fan K-T, Cohen JD, Gray WM (2009) Arabidopsis IAR4 modulates auxin response by regulating auxin homeostasis. Plant Physiol 150:748–758
Seo M, Akaba S, Oritani T, Delarue M, Bellini C, Caboche M, Koshiba T (1998) Higher activity of an aldehyde oxidase in the auxin-overproducing superroot1 mutant of Arabidopsis thaliana. Plant Physiol 116:687–693
Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie D-Y, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191
Stomp A-M (1991) Histochemical localization of b-glucuronidase. In: Gallagher SR (ed) GUS protocols. Academic Press, London, pp 103–113
Sztein AE, Ilić N, Cohen JD, Cooke TJ (2002) Indole-3-acetic acid biosynthesis in isolated axes from germinating bean seeds: the effect of wounding on the biosynthetic pathway. Plant Growth Regul 36:201–207
Tam YY, Normanly J (1998) Determination of indole-3-pyruvic acid levels in Arabidopsis thaliana by gas chromatography–selected ion monitoring-mass spectrometry. J Chromatogr 800:101–108
Tao Y, Ferrer J-L, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, Cheng Y, Lim J, Zhao Y, Ballaré CL, Sandberg G, Noel JP, Chory J (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133:164–176
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Widholm JM (1981) Utilization of indole analogs by carrot and tobacco cell tryptophan synthase in vivo and in vitro. Plant Physiol 67:1101–1104
Wright AD, Sampson MB, Neuffer MG, Michalczuk L, Slovin JP, Cohen JD (1991) Indole-3-acetic acid biosynthesis in the mutant maize orange pericarp, a tryptophan auxotroph. Science 254:998–1000
Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory C (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291:306–309
Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JE, Normanly J, Chory J, Celenza JC (2002) Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev 16:3100–3112
Acknowledgments
We thank Silvia Heinze for technical assistance and Annett Kohlberg and Tilo Lübken for graphical assistance. This work was partially supported by a grant of the ‘Exzellenznetzwerk Biowissenschaften’ from the state Sachsen-Anhalt to MQ and by the U.S. National Science Foundation NSF2010 grant MCB0724970 and USDA-NRI grant 2005-35318-16197 to JDC. Furthermore, helpful suggestions of two anonymous reviewers are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ludwig-Müller, J., Denk, K., Cohen, J.D. et al. An Inhibitor of Tryptophan-Dependent Biosynthesis of Indole-3-Acetic Acid Alters Seedling Development in Arabidopsis . J Plant Growth Regul 29, 242–248 (2010). https://doi.org/10.1007/s00344-009-9128-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-009-9128-1