Abstract
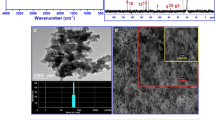
In order to synthesize an improved adsorbent for heavy metal ions, we studied the condensation reaction of chitosan with salicylaldehyde in ethanol to form a Schiff base. The effect irradiating the reaction using an ultrasonic liquid processor was contrasted with conventional methods. The IR spectra of condensed chitosan prepared by the two methods showed that their molecular structures were identical. The reaction conditions, including solvents, ultrasonic power density and irradiation time, pH, and reactant ratio, were optimized by orthogonal design. A shorter reaction time and a higher product yield were obtained using ultrasonic-assisted synthesis compared with the traditional method. A condensation degree of 89.63% was achieved using the optimized conditions: i.e. ultrasonic irradiation at 180 W for 60 min; 95% ethanol as the solvent, pH 4.0, and salicylaldehyde:chitosan ratio of 6:1.
Similar content being viewed by others
References
Chan P, Kurisawa M, Chung J E, Yang Y Y. 2007. Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials, 28: 540–549.
Crini G, Badot P M. 2008. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Progress in Polymer Science, 33: 399–447.
Drofenik M, Kristl M, Makovec D, Jagličić Z, Hanžel D. 2008. Sonochemically assisted synthesis of zinc-doped maghemite. Ultrasonics Sonochemistry, 15: 791–798.
Ghasemi S, Mousavi M F, Shamsipur M, Karami H. 2008. Sonochemical-assisted synthesis of nano-structured lead dioxide. Ultrasonics Sonochemistry, 15: 448–455.
Jayakumar R, Nwe N, Tokura S, Tamura H. 2007. Sulfated chitin and chitosan as novel biomaterials. International Journal of Biological Macromolecules, 40: 175–181.
Kim I Y, Seo S J, Moon H S. 2008. Chitosan and its derivatives for tissue engineering applications. Biotechnology Advances, 26: 1–21.
Kim T H, Jiang H L, Dhananjay J. 2007. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Progress in Polymer Science, 32: 726–753.
Krajewska B. 2005. Membrane-based processes performed with use of chitin/chitosan materials. Separation and Purification Technology, 41: 305–312.
Ma G. 2008. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydrate Polymers, 74: 121–126.
Majeti N V, Ravi K. 2000. A review of chitin and chitosan applications. Reactive & Functional Polymers, 46: 1–27.
Manuel G, Antonio S N, Maria E M. 1992. A derivative of chitosan and 2,4-pentanedione with strong chelating properties. Carbohydrate Research, 233: 255–259.
Neppolian B, Wang Q, Jung H, Choi H. 2008. Ultrasonic-assisted sol-gel method of preparation of TiO2 nano-particles: Characterization, properties and 4-chlor-ophenol removal application. Ultrasonics Sonochemistry, 15: 649–658.
Qu R, Ma Q, Zheng X, Sun Y. 1995. Preparation of chitosan derivatives and their adsorption behavior. Chinese Journal of Applied Chemistry, 12: 117–118.
Pasanphan W, Chirachanchai S. 2008. Conjugation of gallic acid onto chitosan: An approach for green and water-based antioxidant. Carbohydrate Polymers, 72: 169–177.
Perrin D D, interpreted by Shi Y. 1987. Purified methods of chemical leechdom in laboratory, Chemical industry publishing company. 320p.
Qu R, Zheng X, Sun Y. 1995. Preparation of chitosan derivatives and their adsorption behavior. Chinese Journal of Applied Chemistry, 12: 117–118.
Rinaudo M. 2006. Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31: 603–632.
Schuetz Y B, Gurny R, Jordan O. 2008. A novel thermoresponsive hydrogel based on chitosan. European Journal of Pharmaceutics and Biopharmaceutics, 68: 19–25.
Sinha V R, Singla A K, Wadhawan S, Kaushik R, Kumria R, Bansal K, Dhawan S. 2004. Chitosan microspheres as a potential carrier for drugs. International Journal of Pharmaceutics, 274: 1–33.
Stavarache C, Vinatoru M, Maeda Y. 2007. Aspects of ultrasonically assisted transesterification of various vegetable oils with methanol. Ultrasonics Sonochemistry, 14: 380–386.
Tian X L, Liu Z. 1997. Study of condensation chitosan by salicylaldehyde. Chinese Journal of Guangxi Normal University, Supplement: 268–272. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Doctoral Discipline Special Foundation, Education Ministry of China (No. 20050561014), and the National High Technology Research and Development Program of China (863 Program) (No. 2007AA100405)
Rights and permissions
About this article
Cite this article
Duan, L., Guo, S., Yang, J. et al. Ultrasonic-assisted condensation of chitosan with salicylaldehyde. Chin. J. Ocean. Limnol. 27, 882–886 (2009). https://doi.org/10.1007/s00343-009-9204-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-009-9204-1