Abstract
In this work, the UV absorption cross sections of the fluorescence tracer 1-methylnaphthalene are determined in the range of 230–330 nm. The experiments are performed in a continuously scavenged gas flow cell, which allows for defined homogeneous conditions regarding temperature, pressure, and tracer/fuel composition. A LSDS (laser driven light source) is used for irradiation, which enables high spectral emission intensities in the UV range studied. For detection, a spectrograph in combination with an intensified camera is applied. Absorption cross sections at temperatures up to 850 K are determined and compared to sparsely available published data. Possible uncertainties caused by the optical setup and the flow cell, respectively, are considered.
Similar content being viewed by others
1 Introduction
Polycyclic aromatic hydrocarbons (PAH) such as 1-methylnaphthalene (1-MN) play an important role in the field of combustion technology. As a soot precursor, the experimental investigation of PAH can contribute to understand the soot formation process. As both PAH and soot have a high toxicological potential, the concentration in the flue gas should be kept as low as possible, also in order to meet the required emission limit values. Absorption spectroscopy is a suitable measurement technique for the investigation of optical properties of PAH. Several PAH, including naphthalene, have already been studied by using UV–VIS absorption spectroscopy [1]. Additionally, PAH such as 1-MN [2,3,4,5,6], 2-MN [4], naphthalene [6,7,8], dimethylnaphthalene [8], anthracene [9], and perylene [10] are applied as laser induced fluorescence (LIF) tracers for mixing studies in gas flows and combustion processes. The LIF technique can be used for the determination of temperature and fuel distribution, thus contributing to the optimization of the combustion process and to the reduction of pollutant emissions. To enable a quantitative determination of the parameters of interest, a calibration of the fluorescence behavior of the tracer is necessary. Since the fluorescence signal is directly proportional to the absorption cross section σabs, this quantity must be determined to gain a precise understanding of the fluorescence characteristics. For 1-MN only few and inconsistent UV absorption data is available and further experiments are necessary for quantitative prediction and modeling of fluorescence emissions. For example, Suto et al. [11] studied, among others, the absorption behavior of naphthalene and its derivatives 1-MN and 2-ethylnaphthalene from 190 to 295 nm at 297 K using synchrotron radiation. For 1-MN a value of ~ 1.8∙10–17 cm2 at 266 nm, which is the typical excitation wavelength for aromatic LIF tracers, is given. Orain et al. [8] showed a non-monotonic function of the temperature-dependent absorption cross section of naphthalene from 350 to 900 K at 266 nm. For naphthalene and its derivatives 1-MN and 1,3-dimethylnaphthalene a value of 1.28∙10–17 cm2 is given at 350 K and 266 nm. Baranger [12] showed nearly temperature independent values of 7.2∙10–18 cm2 for naphthalene, 7.5∙10–18 cm2 for 1,3-dimethylnaphthalene, and 4.1∙10–18 cm2 for 1-MN from 440 to 620 K at 266 nm. It should be noted that these values are one order of magnitude lower than in the other two references [8, 11].
The fluorescence signals of 1-MN have been calibrated in a wide pressure and temperature range for varying mixtures with oxygen. This calibration data serves for the determination of temperature and fuel distribution using 2-color LIF and FAR-LIF (fuel–air ratio) [3, 4, 6, 13]. However, no detailed temperature-dependent analysis of the absorption behavior has been performed yet, which is necessary to explain the observed non-monotonous fluorescence calibration functions [3]. For this purpose, the temperature dependent absorption cross section of 1-MN is analyzed from 350 to 850 K in a spectral range of 230–330 nm in this experimental study.
2 Experiment
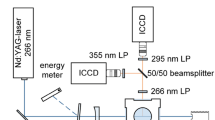
The experiments are performed in a continuously scavenged flow cell (HTC2-high temperature calibration cell) at homogenous conditions (Fig. 1).
To allow evaporation of the tracer at temperatures as low as 350 K and to avoid complete absorption of the light, a gas mixture of 1 vol. % 1-MN (Merck Millipore, purity ≥ 94%) in isooctane (Rotisolv® UV/IR grade, purity ≥ 99.8%) is added to the gas flow entering the cell. The tracer/fuel mixture is adjusted and evaporated by a controlled evaporating and mixing system (CEM, Bronkhorst, not shown in Fig. 1). After the heating section, the carrier gas (dry compressed ambient air) and nitrogen (Linde, nitrogen 5.0, purity 99.999%) and the vaporized 1-MN/isooctane flow are combined in the mixing section of the cell. The volume flow rate of the carrier gas is adjusted by mass flow controllers (MFC, Bronkhorst). During all measurements, the molar concentration of the fuel/tracer is kept constant at 0.5 mol/m3 in the measurement volume. Depending on the measurement point, the residence time of the mixture at elevated temperature varies between 116 and 283 ms. The residence time is calculated from the known total volume flow rate and the distance between the mixing zone and the center of the LIF measurement plane in the cell. Specially designed windows and flanges guarantee a constant diameter of the flow duct over the entire measuring volume, thus minimizing cavities that lead to recirculation zones and temperature gradients. The stepped quartz glass windows (Sico, SQ1 quality, transmission of 0.96 at 200 nm) have a thickness of 15 mm and an inner diameter of 7.5 mm. The absorption path between both windows is 24.6 mm. The studied temperature ranges are 350–850 K being varied in steps of 50 K. Additional experiments using broadband supercontinuum laser absorption spectroscopy (SCLAS) were performed to investigate the fuel/tracer conversion by detection of the produced H2O molecule (not shown here, this will be presented in a forthcoming paper). The results do not show any indication of tracer decomposition and oxidation at maximal temperature conditions (equivalence ratio of Φ = 1 up to 850 K). To be completely sure, for temperatures higher than 500 K, all measurements were performed in pure nitrogen atmosphere. From 350 to 500 K, dry air is used as carrier gas. Control measurements at 500 K did not show any influence of the carrier gas on the absorption behavior of 1-MN. Prior to the experiment, a temperature field analysis in the cell was performed with three thermocouples (type K, TC Direct, 1 mm) at a distance of 7 mm along the absorption path. A maximum temperature gradient of 3.5% was determined between the three thermocouples at 750 K, while the average temperature gradient over all operation points was 2.1%. An average temperature determined by the three thermocouples is used as reference for the absorption measurements. The pressure was measured with a pressure transducer (PAA-33X/80794, Keller). The pressure is kept constant at 0.4 MPa for all operation points, which corresponds to the minimum pressure at which the required volume flows can still be adjusted. Uncertainties depending on temperature, molar concentration of the tracer as well as the influence of pressure are presented at the end of the manuscript. For irradiation, a Laser-Driven Light Source (LDLS, ENERGETiQ EQ-99X-FC) is used, which provides broadband emission from 190 to 2100 nm. After the fiber exit, the light is first collimated (6 mm) by an aluminum coated parabolic mirror (Newport, 50328AL), passed through the measuring volume and then focused by a cylindrical concave UV-enhanced aluminum mirror (Thorlabs, CCM254-100-F01) onto the entrance slit of the spectrograph (Acton SP2300, slit: 5 µm). A 300 l/mm grating with a blaze wavelength of 300 nm is applied. The spectrally resolved signal is then detected by an intensified CCD camera (DiCAM pro 12 bit, exposure time: 100 µs, spectral resolution: 1.15 nm, quantified with a Hg(Ar)-lamp at 253.7 nm). Additional measurements are performed using a commercially available spectrometer (Ocean Optics, Ocean FX, CMOS detector, exposure time: 60 µs, slit: 50 µm, spectral resolution: 1.08 nm) to investigate the effect of the detection system on the results. More information about the LIF-Tracer 1-MN and the calibration cell can be found in references [3, 5, 13].
For every operation point, six absorption measurements (I) of 377 images each (limited by the internal memory of the camera) are performed to minimize the influence of potential temporal fluctuations and spatial inhomogeneities. Directly before and after these measurements, three reference measurements (I0) with 377 images each are performed. Out of these images, a mean vector for I and I0 covering the spectral range considered is calculated within a region of interest (ROI). The ROI has a height of 50 pixels, covering the main part of the beam diameter. I0-measurements are done at the same conditions as the I-measurements but in pure air/nitrogen without the fuel/tracer flow. The absorption cross section is then determined based on these I/I0-vectors using the Beer-Lambert law. This procedure is repeated at three different days. Additional I0-measurements with isooctane at the same molar concentration as in the I-measurements did not show any influence on the determined absorption spectrum in the relevant spectral range of 230–330 nm.
3 Results
All the results presented below show the mean value of the three measurements (each consisting of six individual measurements) performed at three different days. Figure 2 exhibits the temperature dependent absorption spectra of 1-MN at 0.4 MPa between 350 and 850 K in the range of 230–330 nm.
At 350 K, clear spectral structures with three peaks at 264 nm, 274 nm, and 285 nm are visible. A local maximum of the absorption cross section of about 3∙10–17 cm2 is found at 350 K and 274 nm. With increasing temperature, the structures are less distinct and the spectra are broadened and red-shifted. According to the Boltzmann distribution, the probability of reaching higher vibrational levels within the electronic ground state increases with increasing temperature. Therefore, the energy gap between the singlet states S0 → S1 decreases, which can explain the spectral red-shift of the absorption spectra with increasing temperature. The convolution of each spectrum with the underlying Maxwell–Boltzmann distribution kernel for elevated temperature smears out single peaks and the spectra become less structured. With increasing temperature, more vibrational and rotational levels are occupied. Additionally, the energy gap between vibrational levels decreases as the vibrational level increases with temperature. Furthermore, Doppler broadening increases with temperature. At temperatures higher than 800 K the “red part” of the S0 → S2 transition (< 240 nm) could be significant and thus overlaps with the S0 → S1 transition. This would lead to increasing absorption cross-sections in the considered spectral range from 230 to 330 nm. Increasing absorption cross-sections at high temperatures were also found for naphthalene [8] (as discussed below) and toluene [14].
The spectral shift leads to increased absorption cross sections with increasing temperature at wavelengths above ~ 287 nm. The peak maximum slightly shifts from 274 nm at 350 K to 275 nm at 800 K/850 K. Less distinct spectral features and spectral broadening with increasing temperature were also observed for naphthalene [15]. Compared to naphthalene, a red-shift of the 1-MN absorption spectra of approximately five nanometers is found (at constant temperature). In general, the absorption spectrum of 1-MN shows less distinct structure than that of naphthalene [11, 15]. Since 1-MN is usually excited at 266 nm in tracer-LIF measurements [3,4,5, 13], Fig. 3 shows the course of the absorption cross section with the temperature at this wavelength.
From 350 to 800 K the peak maximum at 274 nm, shown in Fig. 2, decreases. However, due to the above-mentioned less distinct structures with increasing temperature, the absorption cross section at 266 nm stays almost constant between 350 and 500 K. A maximum cross section of 2.45∙10–17 cm2 is found at 450 K. From 500 to 800 K, the absorption cross section decreases by about 20%. From 800 to 850 K, the mean value of the absorption cross-section increases again. The large error bars at 850 K already indicate that this non-monotonous behavior cannot be clearly proven. The absorption cross section increases with a probability of 62%. However, a possible physical reason is given above and similar results were already shown for naphthalene: there, due to the spectral features, the absorption cross section first increases up to 500 K, then decreases up to 850 K, and then increases again from 850 to 900 K [8]. In contrast to this result, Zhang et al. [16] showed a linear increase of the absorption cross section of naphthalene at 266 nm from 673 to 1373 K.
The error bars exhibited in Fig. 3 correspond to the 95% confidence interval over three values determined on three different days. The confidence interval was calculated using the student’s t-distribution (t-value: 4.303, 2.48). Between 400 and 800 K, the individual measurements were very similar with a deviation of 2.2%. At 350 K and 850 K, the 2.48 value is significantly higher with values of 9.0% and 13.1%, respectively. The increased standard deviation at 350 K may be explained by a higher variation of the tracer concentration as the fuel/tracer mixture cannot be completely evaporated in the CEM-system (due to the low vapor pressure). At 850 K, the calibration cell operates at its flow limit, which can cause fluctuations of the mass flow and inhomogeneities. The standard deviation at 266 nm is a representative value for all measurement points.
In the following, an error estimation will be given including a comparison with literature data and a discussion about the influence of the detection system on the results. Figure 4 shows the result of this work at 350 K in comparison to the available literature data of Suto et al. [11] in the range of 230–295 nm at 297 K and Orain et al. [8] at 266 nm and at 350 K.
A comparison of the absorption spectrum with data of Suto et al. shows a good agreement regarding the positions of the three spectral features (spectral shift at 274 nm: ~ 0.5 nm) and the relative height of the individual peaks. The relative height of the peaks in Suto et al. is slightly increased and the peaks are more distinct, which could be explained by the lower temperature. In the present study, measurements below 350 K have not been possible as the CEM-system does not enable complete evaporation of the fuel/tracer mixture due to the absolute pressure of 0.4 MPa (Suto et al.: < 1.33∙10–6 MPa). Between 230 and 286 nm, the absolute values of this work are higher than those reported by Suto et al. (at 274 nm ~ 0.5∙10–17 cm2). Between 286 and 295 nm, a good agreement of the absorption cross sections is found. Orain et al. [8] determined a value of 1.28∙10–17 cm2 at 350 K and 266 nm for 1-MN, naphthalene and 1,3-dimethylnaphthalene, which is significantly lower than the value of this work (2.4∙10–17 cm2). It should be noted that, due to significant differences in the spectral structure, it is unlikely that all three substances have exactly the same absorption cross section under the same conditions.
Additionally, the deviation in absorption cross sections introduced by the detector is considered. Figure 5 shows a comparison of two detection systems (Acton SP2300 + DiCAM Pro and Ocean Optics FX) at 350 K, 550 K, and 750 K.
For both detection systems, the optical setup and the experimental parameters were the same. For all temperatures the spectral position and shape is almost identical. For 550 K and 750 K also the absolute values of the absorption cross sections are hardly influenced by the detection system. At 350 K for wavelengths up to 286 nm, the absolute values determined by the ocean optics spectrometer are slightly increased. At 274 nm, the absorption cross sections determined by OO are 9% higher. However, as discussed above, the uncertainty at this temperature is also increased. We conclude that the deviations are mainly introduced by the flow cell, which lead to large variations. The spectral resolution of the Acton SP2300 + DiCAM-system is reduced by the intensifier. This results in almost the same resolution as the OO-system despite the much smaller slit size (5 µm in comparison to 50 µm for OO). The comparison shows that the detection chip (ICCD or uncooled CMOS) does not influence the results. Furthermore, the influences of fuel/tracer concentration and the absolute pressure are investigated (not shown here). Basically, these parameters variations could introduce differences in the flow and mixing behavior in the cell. The pressure influence on the results is negligibly low. The absorption cross sections determined at 1.2 MPa differ on average by 1.6% from the results at 0.4 MPa (at 274 nm and 500 K). By reducing the molar concentration of the fuel/tracer from 0.5 mol/m3 to 0.375 mol/m3, the results differ by 2.6% (at 274 nm and 500 K), which is lower than the maximum error for the MFC-adjusted tracer concentration in the cell (see below).
Finally, an estimate on the possible main sources of error is given. Errors are introduced either by the components of the flow cell itself or by the optical setup. The MFCs for adjusting the air/N2 volume flow, the CEM system and the temperature as well as pressure transducer and the fluctuation of the LDLS contribute to a maximum error of 4.4% for the absorption cross section. This error was calculated by means of an error propagation. The individual uncertainties are provided in Table 1. The fluctuation of the LDLS is 1.0% (mean value of the 95% confidence interval of 18 (six measurements on 3 days each) averaged I0 vectors in the considered spectral range from 230 to 330 nm. One I0 vector consists of 377 reference measurements without tracer flow but gas flow at 0.4 MPa and at 350 K. To minimize the influence of the LDLS fluctuations, all signals I and I0 are normalized to the signal intensity at ~ 390 nm (as no absorption takes place at this wavelength). In addition, the temperature gradient along the absorption path must be taken into account. Here, between 350 and 850 K, an average temperature gradient of 2.1% between the three thermocouples was found (as mentioned above). The linearity of the camera is not taken into account as the absorption signal I is normalized to the reference signal I0.
4 Conclusion
In this work, the absorption cross sections of 1-MN from 230 to 330 nm were determined for temperatures from 350 to 850 K. A laser driven light source was used for broadband emission, which provides high signal intensities in the UV range. A spectrograph in combination with an ICCD camera was applied as detection system. With increasing temperature, characteristic features within the absorption spectra of 1-MN become less distinct and the spectra are broadened and red-shifted. At 266 nm, the absorption cross section nearly stays constant between 350 and 500 K and then decreases up to 800 K. Finally, it tends to increase again from 800 to 850 K, which could be explained by an overlap of the of the S0 → S2 transition (< 240 nm) with the S0 → S1 transition. A comparison with the results of a commercially available spectrometer showed only small deviations. An error estimation was conducted, which showed that the components of the flow cell introduced the largest uncertainty of the absorption cross sections.
The work can help for an improved understanding of LIF calibration data of the tracer 1-MN for the investigation of the mixture formation, e.g., in diesel combustion processes. Furthermore, 1-MN as a soot precursor in reactive flows can be investigated using LIF and absorption spectroscopy with the help of this work and contribute to the understanding of the soot formation process.
References
A. Tregrossi, B. Apicella, A. Ciajolo, C. Russo, Chem. Eng. Trans. 57, 1447 (2017)
F. Payri, J.V. Pastor, J.M. Pastor, J.E. Juliá, Int. J. Eng. Res. 7, 77 (2006)
U. Retzer, W. Fink, T. Will, S. Will, L. Zigan, Appl. Phys. B 125, 124 (2019)
J. Trost, L. Zigan, A. Leipertz, D. Sahoo, P.C. Miles, Appl. Opt. 52, 8001 (2013)
J. Trost, L. Zigan, A. Leipertz, D. Sahoo, P.C. Miles, Int. J. Eng. Res. 15, 741 (2014)
S.A. Kaiser, M.B. Long, Proc. Combust. Inst. 30, 1555 (2005)
S. Faust, G. Tea, T. Dreier, C. Schulz, Appl. Phys. B 110, 81 (2013)
M. Orain, P. Baranger, B. Rossow, F. Grisch, Appl. Phys. B 102, 163 (2011)
J.K. Yoon, K.J. Myong, J. Senda, H. Fujimoto, J. Mech. Sci. Technol. 23, 2565 (2009)
F.P. Hindle, T.L. Yeo, K.B. Ozanyan, N.R.J. Poolton, H. McCann, IEEE Sens. J. 3, 766 (2003)
M. Suto, X. Wang, J. Shan, L.C. Lee, J. Quant. Spectrosc. Radiat. Transf. 48, 79 (1992)
P. Baranger, Kerosene Detection Using Laser Induced Fluorescence Imaging for Aeronautical Engines Application (Université Paris Sud, Paris, 2004)
S. Lind, U. Retzer, S. Will, L. Zigan, Proc. Combust. Inst. 36, 4497 (2017)
W. Koban, J.D. Koch, R.K. Hanson, C. Schulz, Phys. Chem. Chem. Phys. 6, 2940 (2004)
H. Grosch, Z. Sárossy, H. Egsgaard, A. Fateev, J. Quant. Spectrosc. Radiat. Transf. 156, 17 (2015)
Y. Zhang, L. Wang, P. Liu, Y. Li, R. Zhan, Z. Huang, H. Lin, Appl. Phys. B 125, 6 (2018)
Acknowledgements
Open Access funding provided by Projekt DEAL.
Funding
This study was funded by Deutsche Forschungsgemeinschaft (DFG Zi 1384/3) and Erlangen Graduate School of Advanced Optical Technologies (SAOT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Retzer, U., Ulrich, H., Bauer, F.J. et al. UV absorption cross sections of vaporized 1-methylnaphthalene at elevated temperatures. Appl. Phys. B 126, 50 (2020). https://doi.org/10.1007/s00340-020-7400-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-020-7400-z