Abstract
A transparent composite sample containing BaTiO3 was synthesized by using the melting quenching method. The presence of a glassy phase in this composite sample was detected through XRD analysis, and this was further confirmed by DSC study. TEM and SAED analyses provided evidence that the BaTiO3 nanoparticles/clusters are embedded within the borate glass matrix, establishing the composite nature of this sample. FTIR spectrum of the present sample revealed that the glass matrix is composed of two structural groups (BO3 with NBO’s, and BO4), along with the distinct groups for BaTiO3. XPS spectra of the present composite sample indicated the presence of more than one type of boron, barium, titanium and oxygen. DSC and dielectric studies of the present composite sample revealed the presence of the phase transition temperature (Tc). Dielectric constant (ɛr) and dielectric loss (tan δ) curves of the present composite sample displayed an anomaly peak in the vicinity of TC. The optical transmission spectrum of the present composite sample exhibit two transmission bands of Ti3+ (3d1) ions in tetragonal distorted sites. At room temperature, the present transparent composite sample exhibited double hysteresis loops for BaTiO3 at low electric fields. The results obtained can be used for the development of lead-free ferroelectric material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, transparent composite containing BaTiO3 have attracted much attention because of their promising applications in electronic and optoelectronic devices [1,2,3,4]. These composite materials exhibit promising characteristics which due to the presence of two constituents namely the BaTiO3 nanoparticles and the glass matrix [2, 3]. The change of the ferroelectric behavior of ABO3 (such as SrTiO3 and BaTiO3) in the amorphous matrix or glass matrix arises from the strain induced during the growth of nanoparticles within the host matrix [5, 6]. This strain within the amorphous matrix or glass matrix causes the TiO6 octahedra to rotate in opposite directions concerning their neighboring octahedra [7]. With the ongoing trend towards the miniaturization of electronic devices, the reduction of the particle size of ferroelectric materials embedded in a glass matrix has a significant impact on their polarization and phase transition temperatures [5, 6]. BaTiO3 is one of the foremost ferroelectric materials because of its technological importance. When BaTiO3 nanoparticles embedded in the glass matrix, they give unconventional properties [1, 4]. Merz reported that the double hysteresis loop (P-E) for BaTiO3 was observed near Curie temperature as well as the shape and the size of these loops was changed with increasing the temperature [8]. The double hysteresis loop (P-E) can result from antiferroelectric materials, electric field induced near Curie temperature, and/or aging effect below Curie temperature [9]. In the case of aged Mn-doped BaTiO3 single crystal, the double hysteresis loop (P-E) arises due to recoverable electro-strain [10, 11]. Shebanov et al. reported that the transition between antiferroelectric and ferroelectric phases can be induced by electric field, temperature variation, or mechanical stress [12].
Among various host matrices, borate glasses (B2O3) have been widely used because of their advantageous properties such as good glass forming ability, low melting point, high transparency, high thermal stability, as well as have a large variety of structural units (diborate, triborate, and tetraborate) [1, 13]. Therefore, the solubility of the BaTiO3 ferroelectric nanoparticles in borate glasses is high [14]. Abdel-Khalek et al. investigated the (100 ̶x)Li2B4O7 ̶xBaTiO3 (where x = 0, 20, and 60 mol%) and (30–x)V2O5–xBaTiO3 ̶ 70TeO2 (where x = 5, 10, and 15 mol%) systems and they found that the BaTiO3 ferroelectric nanoparticles are formed within glass matrices during the glass formation via the melt-quenching method [1, 3]. Furthermore, the ferroelectric-paraelectric transition temperature (TC) of BaTiO3 nanoparticles in glass matrix was identified through dielectric studies. Singh et al. investigated the addition of BaTiO3 into bismuth borate glasses containing 1Dy2O3 and observed the gradual conversion of BO3 into BO4 units, along with a decrease in the optical band gap energy as the BaTiO3 content increased [4]. Despite numerous studies on composites containing BaTiO3 ferroelectric nanoparticles, there has been notable absence of investigation regarding the polarization-electric field (P-E) characteristics of the BaTiO3 nanoparticles embedded in a borate glass matrix. It is well known that P-E loop is used to characterize a ferroelectric material which is considered as the fingerprint for the information on the structure and properties of this material [15]. In the present investigation, we have fabricated BaTiO3 nanoparticles embedded in borate glass matrix with the composition 25 BaTiO3 ̶ 75 B2O3 (in mol %). The structural, thermal, optical, and P-E properties of this sample have been investigated.
2 Experimental procedures
A transparent composite sample with the composition of 25 BaTiO3 ̶ 75 B2O3 (in mol %) was prepared by using the melting quenching method. The raw materials employed in the preparation of this composite were high-purity B2O3 (99.99%), and BaTiO3 (99.99%). These raw materials were initially ground in an agate mortar and subsequently melted in a porcelain crucible at a temperature of 1150 °C for one hour using an electric furnace. The resultant molten materials were expeditiously poured and quenched onto a copper plate and immediately pressed into plates at room temperature to obtain this composite sample. X-ray diffraction (XRD) pattern of the present transparent composite sample was carried out using a Bruker Co D8 Discover X-ray diffractometer with Cu Kα radiation of wavelength 1.54 Å operating at 40 kV and 40 mA. The transmission electron microscopy (TEM), selected area electron diffraction (SAED), and high-resolution transmission electron microscopy (HR-TEM) studies of the present transparent composite sample were carried out using a JEOL model JEM-2100 PLUS with LaB6 source operating at an accelerating voltage of 200 kV. The energy dispersive X-ray spectroscopy (EDS) elemental mapping analysis of the present transparent composite sample were performed on a JEM-2100 F (URP) instrument operating at 200 kV and equipped with Dry SD30GV Detector. Fourier transform infrared (FTIR) spectrum of the present transparent composite sample was recorded using a Thermo Scientific Nicolet iS50 FT-IR spectrometer with KBr pellets. The FTIR spectrum data were collected in the wavenumber range of 400–1800 cm−1, with a spectral resolution of 4 cm−1 at room temperature. X-ray photoelectron spectroscopy (XPS) of the present transparent composite sample was carried out using a Thermo ScientificTM K-AlphaTM USA with a monochromatic AL-Kα radiation source. Differential scanning calorimetry (DSC) of the present transparent composite sample was carried out using a TA Instrument STA model 650 in the temperature range from 30 to 1100 °C at a heating rate of 15 °C/min. The dielectric constant (εr) and dielectric loss (tan δ) as a function of temperature at different frequencies ranging from 1 to 100 kHz for the present composite sample was measured by using an electronic RLC bridge type SR 720. Optical transmission spectrum of the present transparent composite sample, in the wavelength range 190–824 nm, was carried out using a Hitachi 6405 spectrophotometer. The polarization-field (P-E) loops at various electric field of the present transparent composite sample were recorded at room temperature. This measurement was carried out using a ferroelectric tester (Precision premier II, Radiant technologies Inc., USA) at a frequency of 10 Hz.
3 Results and discussion
3.1 XRD, TEM, SAED, and HR-TEM studies
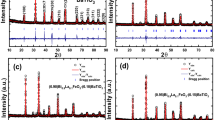
Figure 1 displays the XRD pattern of the present transparent composite sample at room temperature. This pattern is characterized by broad humps without any distinct sharp Bragg peaks for crystalline BaTiO3. This feature suggests the presence of amorphous nature (glassy phase) of the present transparent composite sample. The present XRD pattern fails to reveal the crystallinity of the BaTiO3 nanoparticles within the borate glass matrix, mainly due to their small size and low concentration in glass matrix [1, 16]. Figure 2 displays the photograph of the present transparent composite sample. It is noticed that the present sample exhibits transparency and appears yellow when observed at a macroscopic level. This observation provides evidence of the presence of a small amount of Ti3+ in the present sample thus evidence of the incorporation of BaTiO3 nanoparticles within the borate glass matrix [1, 16].
Figure 3a displays the TEM image of the present transparent composite sample, captured in the first selected region. This image clearly displays the BaTiO3 nanoparticles/clusters phase (dark) embedded within a continuous borate glass matrix phase (gray region). This observation leads to the conclusion that the present sample is indeed a glass-nanocomposite, characterized by the presence of two different constituents namely the BaTiO3 nanocrystalline phase and glass matrix. This finding aligns with reported for (100–x) Li2B4O7 ̶xBaTiO3 (where x = 20, and 60 mol%) glass-nanocomposite by Abdel-Khalek et al. [1, 16]. Figure 3b displays the SAED pattern of the present transparent composite sample obtained from Fig. 3a. The SAED pattern consists of diffuse rings or halos, indicating the presence of an amorphous nature (glassy phase) in this sample [17]. Figure 3c displays another TEM image of the present transparent composite sample, captured in the second selected region. Like the previous TEM image, it clearly displays the presence of BaTiO3 nanoparticles phase (dark) embedded within the continuous borate glass matrix (gray region). Figure 3d displays the SAED pattern of the present transparent composite sample obtained from Fig. 3c. This pattern exhibits diffraction rings with some spots surrounding the bright central region. These diffraction rings confirm the presence of BaTiO3 polycrystalline structure [18]. Therefore, it becomes evident from the electron diffraction pattern that the present sample is indeed a glass-nanocomposite, given the coexistence of amorphous (borate glass matrix phase) and crystalline (BaTiO3 nanoparticles) states in this sample.
Figure 4 displays the elemental mapping images of the present transparent composite sample. These images display the distribution of barium (Ba), boron (B), oxygen (O), and titanium (Ti) elements. All these elements are uniformly distributed, and no other elements show up (impurities). This observation underscores the purity of the present glass-nanocomposite sample. To identify the shape and determine the average particle size of BaTiO3 nanoparticles/clusters, a specific area containing these nanoparticles/clusters was magnified, as shown in Fig. 5a. The particles of BaTiO3 (Fig. 5a) exhibit a predominantly spherical shape with some slight agglomeration. The distribution of particle size histograms of the BaTiO3 nanoparticles is shown in Fig. 5b. The histogram has been fitted with a Gaussian distribution, which allows us to determine the average particle size of the BaTiO3 particles. The average particle size was found to be 44.489 nm. This finding indicates that the present sample is indeed a glass-nanocomposite natures, where the particle size of BaTiO3 within the borate glass matrix falls within the nanoscale regime. Figure 5c displays the HR-TEM image captured from the region described in Fig. 3c. In this image, regions with aligned bright planes (lattice fringes), representing the BaTiO3 phase. In contrast, the remaining regions lack these lattice planes (lattice strips), indicating the presence of the borate glass phase [19]. Moreover, the HR-TEM image displays non-uniform lattice fringes with blurry edge, signifying the presence of BaTiO3 nanoparticles within the borate glass matrix [20]. The interplanar spacing (fringes) as estimated from Fig. 5c is approximately 0.34 nm, corresponding to the (100) plane in the tetragonal BaTiO3 structure (JCPDS File No. 812,203) [21, 22]. This observation confirms the presence of tetragonal BaTiO3 in our sample, characterized by high crystallinity of the particles. It’s worth noting that the interplanar spacing in our sample is higher than that found in Refs. [21, 22]. Additionally, the BaTiO3 nanoparticles in our sample exhibit the formation of extended defects, marked with green ovals, as shown in Fig. 5c. These extended defects have an influence on the ordering of oxygen vacancies in the BaTiO3 [23]. Figure 5d displays the SAED pattern taken from the region in Fig. 5c. This pattern exhibits clear spots, indicating the presence of the BaTiO3 perovskite structure with high crystallinity. This serves as confirmation of the presence of BaTiO3 nanoparticles in our sample. To determine the elemental composition of the nanoparticles/clusters embedded within the borate glass matrix, elemental mapping images of the BaTiO3 nanoparticles were obtained from the region in Fig. 5a, as shown in Fig. 6. These images reveal the distribution of barium (Ba), titanium (Ti), and oxygen (O) elements. Additionally, these elements are uniformly distributed, and there are no indications of other elements, affirming the presence of BaTiO3 nanoparticles/clusters embedded in the borate glass matrix.
3.2 FTIR and XPS studies
Figure 7a displays the FTIR absorption spectrum of the present transparent composite sample in the wavenumber range of 400–1800 cm−1 at room temperature. This spectrum exhibits broad bands, which are a result of the overlapping of the individual bands. To resolve and identify the individual bands that are concealed within the broad spectrum, we deconvoluted the FTIR absorption spectrum of the transparent composite sample based on Gaussian type function as shown in Fig. 7b. This deconvolution revealed twelve distinct bands. The band at 1635 cm−1 is assigned to OH bending mode of vibration [23]. The band at 1630 cm−1 is assigned to asymmetric stretching relaxation of the B–O bond in trigonal BO3 units [24]. The band at 1504 cm−1 is attributed to the B–O asymmetric stretching vibration of BO3 units in pyro-borate and ortho-borate groups [25]. The band at 1466 cm−1 is due to the asymmetric vibrations mode involving three non-bridging oxygen (NBOs) in the B–O–B groups [24, 26]. The band at 1401 cm−1 corresponds to the stretching vibration of various network containing six-membered borate groups in BO3 units [23]. The band at 1284 cm−1 is assigned to the B–O–B bond that links neighboring groups to boroxol groups [23, 27]. The band at 1098 cm−1 results from the stretching vibration of BO4 units, including triborate, tetraborate, and pentaborate groups [28]. The band at 1033 cm−1 can be attributed to the B–O stretching vibration of BO4 groups in diborate groups [29]. The band at 916 cm−1 is assigned to the stretching vibration of tetrahedral BO4 units [14]. The band at 693 cm−1 is assigned to the bending vibrations of B–O–B linkages in the borate network or may be assigned to the stretching vibrations of Ti–O bonds in octahedral TiO6 units [3]. This band indicates the presence of Ti4+ at octahedral sites in the glass network [1, 30]. The band at 569 cm−1 results from the TiO6 octahedra deformation mode of BaTiO3 [1]. Finally, the band at 465 cm−1 is attributed to vibrations of Ba2+ metallic cations in BaTiO3 [30]. The presence of this band in the present composite sample may be due to Ti3+ ions in tetragonally distorted octahedral sites, as further confirmed by subsequent optical results [31]. These FTIR results validate the presence of the ferroelectric BaTiO3 nanoparticles phase embedded within the borate glass matrix. Furthermore, the borate glass matrix is composed of the network former BO3 with non-bridging oxygen (NBO’s), and BO4 within the glass network.
Figure 8a–d displays XPS spectra of the B 1s, Ba 3d, Ti 2p, and O 1s core levels of the present transparent composite sample. These spectra confirm the present composite sample is composed of the expected species, namely B, Ba, Ti, and O. The B 1s XPS spectrum of the present transparent composite sample is shown in Fig. 8a. Deconvolution of the B 1s XPS spectrum revealed two individual peaks at 189.71 and 192.55 eV, corresponding to the boron species present [32]. This spectrum closely resembles those previously reported for borate glasses [32, 33]. The B 1s peak at lower binding energy of 189.71 eV is due to the formation of boron with nonbridging oxygen (NBO) in the present sample [33]. The B 1s peak at higher binding energy of 192.55 eV arises from the formation of B2O3 with bridging oxygen (BO) in the present sample [33]. Figure 8b displays the Ba 3d XPS spectrum of the present transparent composite sample, revealing two peaks of 3d5/2 and 3d3/2 at 780.58 and 795.88 eV, respectively. This spectrum closely resembles that reported by Harizanova et al. for strontium barium titanate glass-ceramics [34]. The peak at a binding energy of 780.58 eV, corresponding to 3d5/2 is attributed to Ba2+ ions within the BaTiO3 lattice [35]. The other peak at a binding energy of 795.88 eV, corresponding to 3d3/2 is assigned to Ba2+ ions located at the surface region of BaTiO3 [36]. The Ti 2p XPS spectrum of the present transparent composite sample is shown in Fig. 8c. This spectrum reveals four individua peaks of 2p3/2 and 2p1/2, indicating the presence of different oxidation states of Ti ions [34]. The peaks at binding energies of 459.01 and 461.63 eV correspond to 2p3/2 and 2p1/2 respectively. Additionally, the peaks at binding energies of 464.69 and 472.63 eV also correspond to 2p3/2 and 2p1/2 respectively. The peaks at higher binding energies are attributed to the existence of Ti4+ ions, while those at lower binding energies may be attributed to the presence of Ti3+ ions [34]. The O 1s XPS spectrum of the present transparent composite sample is shown in Fig. 8d. The XPS spectrum of O 1s for the present sample could be deconvoluted into two individual peaks, consistent with previous literature [35, 37]. According to Gong et al. the O 1s XPS spectrum of 7 wt% glass-BaTiO3 exhibits two peaks attributed to bridging oxygen (BO) and nonbridging oxygen (NBO) [35]. The O 1s peak at a binding energy of 531.74 eV corresponds to oxygen in the BaTiO3 lattice [37, 38]. Meanwhile, the O 1s peak at a binding energy of 534.56 eV is attributed to O2- ions originating from oxygen vacancies [36]. The presence of oxygen vacancies in the present transparent composite sample contributes to the existence of Ti3+ ions [38, 39].
3.3 Thermal and dielectric studies
Figure 9 displays the DSC curve of the present transparent composite sample at the heating rate of 15 °C/min. At approximately 30 °C, an endothermic peak is observed, followed by an endothermic dep at 588 °C. These endothermic features represent the Curie temperature (Tc) of the BaTiO3 (ferroelectric to paraelectric) and the glass transition temperature (Tg) respectively [16]. This observation confirms the presence of ferroelectric BaTiO3 nanoparticles within the borate glass matrix, defining it as a glass-nanocomposite [1, 16]. Additionally, the DSC curve further exhibits two exothermic peaks at TP1=706 (less intense) and TP2=761 °C (more intense). These exothermic peaks represent the crystallization process. The presence of two distinct exothermic peaks suggests the crystallization of two different phases [26]. Moreover, the differing intensities of these peaks imply that one of the two mechanisms, either nucleation or growth, dominates the crystallization process [26]. The large difference between Tg and the onset Tp underscores the thermal stability of the present transparent composite material, highlighting its suitability for various applications. The DSC results align well with the observations made through TEM, SAED, and FTIR analyses, collectively confirming the composite nature of the material.
Figure 10 displays the dielectric constant (εr) as a function of temperature at different frequencies in 1–100 kHz range for the present transparent composite sample. It is observed that the values of ɛr are larger at higher temperatures and lower frequencies, indicating the wide range of applications for the present composite [14]. The increase in εr values with increasing temperature is due to the increase in both electronic and ionic polarizability sources [1]. The decrease in εr values with increasing frequency is attributed to the decrease in orientation and ionic polarizability sources [1]. Furthermore, the increase in ɛr values at higher temperatures and lower frequencies may be due to the increase in space charge polarization near the interface between BaTiO3 nancrystallites and the glass matrix [40, 41]. Additionally, the ɛr curves at all studied frequencies display an anomaly peak, attributed to the phase transition temperature (Tc) of the BaTiO3 phase, consistent with DSC results [1]. Figure 11a displays the ɛr as a function of temperature at 1 kHz for the anomaly peak. It is apparent from Fig. 11a that the Tc of BaTiO3 phase is 295 K. The values of TC of BaTiO3 phase in the present composite sample closely align with those reported by Abdel-Khalek et al. [1, 16]. The broad of the anomaly peak may be due to presence of both non-homogeneous and internal stresses in the present composite [1]. According to Xiao et al. the polycrystalline BaTiO3 have phase transition temperatures, are 1432, 130, 5, – 90 °C, corresponding to the transition between hexagonal, cubic, tetragonal, orthorhombic and rhombohedral, respectively [42]. Amongst them, the tetragonal phase (P4mm) is stable at room temperature [42]. This result is consistent with the XRD pattern of the BaTiO3 raw material used in the preparation of the present composite as shown in Ref. [43]. The change in the Tc value of in present composite may be attributed to the lower particle size of BaTiO3 (44.489 nm). In addition, the presence of the strained BaTiO3 nanoparticles because of borate glass matrix [6].
To determine the order of the phase transition, the relationship between the reciprocal of dielectric constant (1/ɛr) and the temperature above Tc follows the Curie-Weiss law.
where C represents the Curie-Weiss constant and T0 signifies the Curie-Weiss temperature. Figure 11b displays the 1/ɛr as a function of temperature at 1 kHz for the present transparent composite sample. From the linear fitting, the values of C, T0 and the difference (Tc ̶ T0) for the present composite sample are determined to be 1.034 × 103, 274 K, and 21 K, respectively. The magnitude of C and T0 closely match the values obtained in the previous studies of BaTiO3 in glass-nanocomposite samples [2, 16]. The fact that the difference (Tc ̶ T0) is larger than zero, indicates that the phase transition in the present composite sample is of the first order [2, 16]. Figure 12 displays the dielectric loss (tan δ) as a function of temperature at different frequencies ranging from 1 to 100 kHz for the present transparent composite sample. It is observed that the behavior of tan δ, exhibiting larger values at both higher temperatures and lower frequencies, is similar to that for the dielectric constant. Moreover, the tan δ curves at all studied frequencies display an anomaly peak in the vicinity of TC. The high value of tan δ at TC can be attributed to the coupling between space charge and ferroelectricity [44]. The presence of space charge in the present composite sample is attributed to the oxygen vacancies in BaTiO3 nanoparticles, contributing to electrical polarization [44]. Beyond TC, the tan δ values decreases with increasing temperature up to approximately 475 K. This decrease can be attributed to the decreasing contribution of ferroelectric domain walls [41]. However, as the temperature rises above 475 K, conduction loss increases while relaxation loss diminishes [3, 23]. At lower frequencies, the higher values of tan δ may be due to the contribution of conduction loss and electron polarization loss while the decrease in tan δ values with increasing frequency may be attributed to electron polarization loss [23].
3.4 Optical and ferroelectric studies
Figure 13 displays the optical transmission spectrum of the present transparent composite sample at room temperature in the wavelength range (200–825 nm). This spectrum exhibits relatively low levels of transparency and have two distinct transmission bands at 614 and 773 nm. These bands are assigned to the 2B2g →2B1g and 2B2g →2A1g octahedral transitions of Ti3+ (3d1) ions located in tetragonal distorted sites, respectively [1]. These bands agree with that reported for 40Li2B4O7–60BaTiO3 (mol%) glass-nanocomposite, 55B2O3–(25–x)ZnF2–10CaF2–10Al2O3:xTiO2 (0 ≤ x ≤ 1) mol%, and LiF-PbO-B2O3 glasses containing different concentration of TiO2 by Abdel-Khalek et al. [1], Lakshmi et al. [31], and Rao et al. [45], respectively. A significant observation is the shift of these bands to longer wavelength in the present transparent composite sample. This shift suggests a weaker ligand field of Ti3+ (3d1) ions, influencing the optical properties of this material [1]. The optical absorption edge (λcut-off) of the present transparent composite sample equals to 348 nm. To determine the optical band gap (Eopt) of the present transparent composite sample, we used the following Mott and Davis relation [46].
where B is a constant independent of energy, hν is the incident photon energy and α(ν) is the optical absorption coefficient. Figure 14a shows the (α hν)1/2 versus hν plot for the present transparent composite sample. The plot demonstrates a linear region, and by extrapolating the linear portion to the point where (α hν)1/2 = zero on the hν axis, the optical band gap (Eopt) of the present transparent composite sample is determined to be 1.875 eV. The value of Eopt is influenced by the ratio of non-bridging oxygen (NBO’s) to bridging oxygen in this material. NBO’s are known to bind excited electrons less tightly than bridging oxygen, thus affecting the optical band gap [1, 47].
To further assess the degree of disorder within the present transparent composite sample, the Urbach energy (also known as the band tail, E0) was determined. The E0 of the present transparent composite sample was calculated by the following relation [48]:
where B is a constant. Figure 14b shows the plot of ln α versus hν of the present transparent composite sample. The Urbach energy E0 for the present transparent composite sample has been determined to be 1.038 eV. This value was calculated based on the reciprocal of the slope of linear portion observed in the ln α vs. hν plot. A high value of E0 in this composite, suggests that weak bonds have been converted into defects within this material, emphasizing the presence of structural disorder and defects that impact its properties [47].
Figure 15 displays the polarization versus electric field (P-E) of the present composite sample at various electric field. It is observed that the P-E loops at low electric field (10, 11, 13, and 14 kV/cm) exhibit double hysteresis loops, as well as an almost linear P-E relationship in the middle section of the hysteresis loop. The double hysteresis loops in the present composite sample could be explained by the presence of random local strain and defect dipoles in the ferroelectric of BaTiO3 lattice [49,50,51]. Additionally, the other source of the double hysteresis loops in this sample is the first-order ferroelectric transition occurring in the temperature range between the Curie-Weiss temperature (T0) and the Curie temperature (Tc) [52]. Therefore, in the first-order ferroelectric transition, double hysteresis loops are observed near Tc, indicating the electric field-induced phase transition from paraelectric to ferroelectric [50]. This finding is confirmed by the aforementioned dielectric results for the present composite sample, where the phase transition in this sample is of the first order. In ferroelectric material, the double hysteresis loops can be explained by the free energy perspective, where they can be induced by applying an external electric field under special conditions [52]. According to Pu et al., the double hysteresis loops in ferroelectric materials can be attributed to random local strain, which leads to the displacement of oxygen octahedra [51]. According to Randall et al., the double hysteresis loops in ferroelectric materials can be due to randomly oriented defect dipoles and/or switching of the first-order ferroelectric transition above Tc [52]. The reversible domain-switching mechanism in BaTiO3 nanoparticles within the glass matrix may be driven by these point defects, which provide a restoring force and allow for substantial recoverable electro-strain [10]. The presence of random local strain and defect dipoles in BaTiO3 nanoparticles via oxygen vacancy diffusion or oxygen octahedral rotations, as confirmed by the aforementioned HR-TEM and XPS results [10, 49]. Despite the double hysteresis loop (P-E) being an exceptional phenomenon in ferroelectric material, it is often observed in antiferroelectric materials [49]. But the presence of a double hysteresis loop (P-E) alone in any material does not provide evidence for the existence of antiferroelectric materials [52]. From the inset of Fig. 15 at 10 kV/cm, it is observed that the polarization is non-zero at zero electric field, thus the double hysteresis loop (P-E) in the present composite sample does not belong to the antiferroelectric type [53]. Therefore, we can conclude that the double hysteresis loop in the present composite sample at room temperature belongs to the ferroelectric type, which aligns with previous findings in BaTiO3 reported by Merz [8]. Merz discovered for the first time the double hysteresis loop of ferroelectric BaTiO3 at the Curie point [8]. Srivastava et al. studied the origin of the double hysteresis loops (P-E) in the ferroelectric BaTiO3 crystal [54]. As the electric field is further increased (at 20, 25, 30, and 40 kV/cm), the hysteresis loops (P-E) narrow down and exhibit nearly linear dielectric behavior within these electric field ranges [15]. This suggests that the P-E loops transform from a double hysteresis loop to normal hysteresis loop with increasing electric field, possibly due to effects induced by the electric field near Curie temperature [10, 12]. This weakness of the double hysteresis loop and its transition to normal hysteresis loop with increased electric field corresponds with findings reported for BiFeO3 ceramic by Yuan et al. [9]. The P-E loop of the transparent composite sample does not saturate (as seen in Fig. 7), which may be attributed to three reasons: the electrical nonpolling of the glassy phase, the interface between the crystal (BaTiO3) and glass, and the stresses applied on BaTiO3 nanoparticles by the surrounding rigid glass matrix [55, 56]. Parameters of the P-E loops for the present transparent composite sample at various electric field are listed in Table1. It is noticed that the maximum polarization (Pmax) increases with increasing the electric field, possibly due to the dielectric charging effect [57]. The lower values of remnant polarization (Pr) in the present transparent composite sample may be a result of the presence of the glassy phase and the smaller crystallite size of BaTiO3 nanoparticles [56]. The values of the coercive field (Ec) and the loop area increase with increasing the electric field. A high Ec value in the present transparent composite sample can lead to electrical breakdown, thus preventing complete saturation [56]. The loop area of the P-E hysteresis loop represents the energy dissipated within the present composite sample. As the electric field increases, charge carriers within the BaTiO3 move along the electric field, resulting in the dissipation of energy within this composite [58].
>
4 Conclusions
In summary, we reported double hysteresis (P-E) loops for BaTiO3 at room temperature in present transparent composite sample and explained the mechanisms of the double hysteresis loop (P-E) as exceptional phenomenon in the first order ferroelectric material. The transparent composite sample has been prepared by the melting quenching method. TEM, SAED, HR-TEM, and FTIR studies were employed to validate the existence of the BaTiO3 nanoparticles within the borate glass matrix. XPS spectra provided evidence of the presence of oxygen vacancies, Ti3+ and Ti4+ ions in the present composite sample. DSC and dielectric studies of the present composite sample revealed the presence of a ferroelectric to paraelectric phase transition of BaTiO3. Dielectric studies provided evidence that the phase transition in this sample is of the first order and the Curie-Weiss law was found to be valid at temperature above Tc. The optical characteristics of the present composite reveal the existence of octahedral transitions (2B2g →2B1g and 2B2g →2A1g) of Ti3+ (3d1) ions in tetragonally distorted sites. The results obtained, such as double hysteresis (P-E) loops for BaTiO3 in present transparent composite sample, can be used for the development of lead-free ferroelectric materials for energy storage applications.
Data availability
All the data are available within this paper.
References
E.K. Abdel-Khalek, E.A. Mohamed, S.M. Salem, F.M. Ebrahim, I. Kashif, Study of glass-nanocomposite and glass–ceramic containing ferroelectric phase. Mater. Chem. Phys. 133, 69–77 (2012)
V. Thakur, A. Singh, R. Punia, M. Kaur, L. Singh, Effect of BaTiO3 on the structural and optical properties of lithium borate glasses. Ceram. Int. 41, 10957–10965 (2015)
E.K. Abdel-Khalek, I.O. Ali, Structural, AC conductivity and dielectric properties of vanado-tellurite glasses containing BaTiO3. J. Non-Cryst Solids. 390, 31–36 (2014)
L. Singh, V. Thakur, R. Punia, R.S. Kundu, A. Singh, Structural and optical properties of barium titanate modified bismuth borate glasses. Solid State Sci. 37, 64–71 (2014)
J.H. Haeni, P. Irvin, W. Chang, R. Uecker, P. Reiche, Y.L. Li, S. Choudhury, W. Tian, M.E. Hawley, B. Craigo, A.K. Tagantsev, X.Q. Pan, S.K. Streiffer, L.Q. Chen, S.W. Kirchoefer, J. Levy, Schlom1. Nature 430, 758 (2004)
E.K. Abdel-Khalek, E.A. Mohamed, I. Kashif, Ferroelectricity of strained SrTiO3 in lithium tetraborate glass-nanocomposite and glass-ceramic. Phys. B 530, 242–250 (2018)
C. Yuan, S. Ye, B. Xu, W. Lei, Strain induced tetragonal SrTiO3 nanoparticles at room temperature. Appl. Phys. Lett. 101, 071909 (2012)
W.J. Merz, Double Hysteresis Loop of BaTiO3 at the Curie Point. Phys. Rev. 91, 513–517 (1953)
G.L. Yuan, Y. Yang, S.W. Or, Aging-induced double ferroelectric hysteresis loops in BiFeO3 multiferroic ceramic appl. Phys. Lett. 91, 122907 (2007)
X. Ren, Large electric-field-induced strain in ferroelectric crystals by point-defect-mediated reversible domain switching. Nat. Mater. 3, 91 (2004)
L.X. Zhang, X. Ren, In situ observation of reversible domain switching in aged Mn-doped BaTiO3 single crystals. phys. Rev. B 71, 17410 (2005)
L. Shebanov, M. Kusnetsov, A. Sternberg, Electric field-induced antiferroelectric-to-ferroelectric phase transition in lead zlrconate titanate stannate ceramics modified with lanthanum. J. Appl. Phys. 76(7), 4301–4304 (1994)
N. Chopra, S. Kaur, O.P. Pandey, G. Sharma, S. Sharma, Physical, optical and structural characterizations of Dy3+-doped lead borate glasses, IOP Conf. Series: Materials Science and Engineering 1114 (2021) 012098
E.K. Abdel-Khalek, E.A. Mohamed, S.M. Salem, I. Kashif, Structural and dielectric properties of (100 – x)B2O3-(x/2)Bi2O3–(x/2)Fe2O3 glasses and glass-ceramic containing BiFeO3 phase. J. Non-Cryst Solids. 492, 41–49 (2018)
X. Tan, Z. Xu, X. Liu, Z. Fan, Double hysteresis loops at room temperature in NaNbO3-based lead-free antiferroelectric ceramics. Mater. Res. Lett. 6, 159–164 (2018)
E.K. Abdel-Khalek, E.A. Mohamed, Shaaban M. Salem, Dielectric and Pyroelectric properties of BaTiO3 embedded in Li2B4O7 Glass Matrix, Ferroelectrics, 473 (2014) 34–44
G. Senthil Murugan, K.B.R. Varma, Lithium borate–strontium bismuth tantalate glass nanocomposite: a novel material for nonlinear optic and ferroelectric applications. J. Mater. Chem. 12, 1426–1436 (2002)
N. Syam Prasad, K.B.R. Varma, Evolution of ferroelectric LiNbO3 phase in a reactive glass matrix (LiBO2–Nb2O5), J. Non-crys. Solids. 351, 1455–1465 (2005)
C. Liu, S. Xie, H. Bai, F. Yan, T. Fu, B. Shen, Jiwei Zhai excellent energy storage performance of niobate-based glass-ceramics via introduction of nucleating agent. J. Materiomics. 8, 763–771 (2022)
Y. Hao, Z. Feng, S. Banerjee, X. Wang, S.J.L. Billinge, J. Wang, K. Jin, K. Bi, L. Li, Ferroelectric state and polarization switching behaviour of ultrafine BaTiO3 nanoparticles with large-scale size uniformity. J. Mater. Chem. C 9, 5267 (2021)
L. Zhao, D. Guo, X. Kang, L. Liang, Z. Yang, Y. Sang, H. Liu, Morphology tuned BaTiO3 ceramic sintering: crystal facet and size distribution. Sci. Adv. Mater. 8, 1200–1207 (2016)
K. Gupta, S. Singh, M. Ceretti, M.S.R. Rao, W. Paulus, Scaling of extended defects in nano-sized brownmillerite CaFeO2.5, Phys. Status Solidi A 210, 1771–1777 (2013)
E.K. Abdel-Khalek, E.A. Mohamed, A. Ratep, S.M. Salem, I. Kashif, Structural, optical and dielectric characterization of niobium lithium tetraborate glasses doped praseodymium. J. Non-Cryst Solids. 441, 58–65 (2016)
D.D. Ramteke, H.C. Swart, R.S. Gedam, Spectroscopic properties of Pr3+ ions embedded in lithium borate glasses. Phys. B 480, 111–115 (2016)
T.S. Rao, N.V. Prasad, M. Shareefuddin, G. Prasad, Indian J. Sci. Technol. 15(17), 839–849 (2022)
E.K. Abdel-Khalek, S.M. Salem, M. Farouk, E.A. Mohamed, I. Kashif, Structural, optical and dielectric properties of glass-nanocomposite. J. Non-Cryst Solids. 357, 864–872 (2011)
C. Gautam, A.K. Yadav, A.K. Singh, A review on Infrared Spectroscopy of Borate glasses with effects of different additives ISRN ceramics. ID 428497, 17 (2012)
I. Ardelean, P. Pascuta, Comparative vibrational study of xFe2O3·(1 – x)[3B2O3·MO] (MO⇒CaO or CaF2) glass systems. Mater. Lett. 58, 3499 (2004)
K. Swapna, A. Mahamuda, S. Srinivasa Rao, T. Shakya, D. Sasikala, G. Haranath, Vijaya, Prakash, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 125, 53–60 (2014)
S. Thakur, V. Thakur, A. Kaur, L. Singh, Study of the crystallization and structural behavior of Bismuth Barium Titanate Glass-ceramics. J. Non-Cryst Solids. 557, 120563 (2021)
N.R. Lakshmi, S. Cole, Influence of TiO2 ions on Spectroscopic Properties of Oxyfluoride Glasses, Materials Today: Proceedings 18 (2019) 192–206
D.A. Hensley, S.H. Garofalini, XPS investigation of lithium borate glass and the Li/LiBO2, Interface. Appl. Surf. Sci. 81, 331–339 (1994)
K.M. Kaky, E. Şakar, U. Akbaba, A.E. Kasapoğlu, M.I. Sayyed, E. Gür, S.O. Baki, M.A. Mahdi, X-ray photoelectron spectroscopy (XPS) and gamma-ray shielding investigation of boro-silicate glasses contained alkali/alkaline modifier. Results Phys. 14, 102438 (2019)
R. Harizanova, M. Pernikov, I. Mihailova, D. Tatchev, G. Avdeev, I. Avramova, C. Rüssel, J. Chem. Technol. Metall. 58, 8–13 (2023)
Y. Gong, W. Deng, W. Zhang, C. Yatongchai, Y. Zou, R.C. Buchanan, Effect of a BaO-CuO-Bi2O3-B2O3 glass flux, and its processing on the dielectric properties of BaTiO3 Ceramics International 41 (2015) 671–680
E.K. Abdel-Khalek, A.A. Askar, M.A. Motawea, M.A. Aboelnasr, H.H. El-Bahnasawy, Study of the influence of synthesis method in BaFeO3–δ perovskite on structural, optical, magnetic and antibacterial properties. Phys. B: Phys. Condens. Matter. 628, 413573 (2022)
L. Srisombat, S. Ananta, B. Singhana, T.R. Lee, R. Yimnirun, Chemical investigation of Fe3+/Nb5+-doped barium titanate ceramics. Ceram. Int. 39, S591–S594 (2013)
M. Wegmann, L. Watson, A. Hendry, XPS Analysis of submicrometer barium titanate powder. J. Am. Ceram. Soc. 87, 371–377 (2004). [3]
C. Miot, E. Husson, C. Proust, R. Erre, Coutures X-ray photoelectron spectroscopy characterization of barium titanate ceramics prepared by the citric route. Residual carbon study. J. Mater. Res. 12, 2388–2392 (1997)
V.U. Rahangdale, V.K. Deshpande, Study of physical properties of PbTiO3 based glass-ceramic with variation of heat treatment duration. Ferroelectrics. 467, 85–98 (2014)
E.K. Abdel-Khalek, M.A. Elsharkawy, M.A. Motawea, E. Elesh, A.T.M. Farag, Dielectric and Thermal properties of Tetragonal PbTiO3 Nanoparticles/Clusters embedded in Lithium Tetraborate Glass Matrix. Silicon. 13, 2993–3002 (2021)
C.J. Xiao, C.Q. Jin, X.H. Wang, Crystal structure of dense nanocrystalline BaTiO3 ceramics. Mater. Chem. Phys. 111, 209 (2008)
E.A. Mohamed, E. Nabhan, A. Ratep, F.M. Hassan, K. Tahoon, Influence of BaTiO3 nanoparticles/clusters on the structural and dielectric properties of glasses nanocomposites. Phys. B 589, 412220 (2020)
E.K. Abdel-Khalek, S.M. Salem, I. Kashif, Synthesis, crystal structure and ferroelectric properties of SrBi2Nb2O9 embedded in a 50% Li2B4O7 glass matrix. J. Electroceram. 29, 171–178 (2012)
P. Nageswara Rao, C. Laxmi Kanth, D. Krishna Rao, N. Veeraiah, J. Quant. Spectrosc. Radiat. Transf. 95, 373 (2005)
N.F. Mott, E.A. Davis, Electronic Processes in Non-Crystalline Materials, 2nd edn. (Clarendon, Oxford, 1979)
E.K. Abdel-Khalek, A.A. Bahgat, Optical and dielectric properties of transparent glasses and nanocrystals of lithium niobate and lithium diborate in borate glasses. Phys. B 405, 1986–1992 (2010)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92, 1324 (1953)
Y. Pu, J. Zhu, X. Zhu, Y. Luo, M. Wang, X. Li, J. Liu, J. Zhu, Xiao. Double hysteresis loop induced by defect dipoles in ferroelectric pb(Zr0.8Ti0.2)O3 thin films. J. Appl. Phys. 109, 044102 (2011)
K. Li, X.L. Zhu, X.Q. Liu, X. Ma, M.S. Fu, J. Kroupa, S. Kamba, X.M. Chen, Electric-field-induced phase transition and pinched P–E hysteresis loops in Pb-free, ferroelectrics with a tungsten bronze structure. NPG Asia Mater. 10, 71–81 (2018)
Y. Pu, D. Liu, X. Shi, Double-hysteresis-like loops in Cr2Ti3O9-doped BaTiO3 ceramics. Vacuum. 99, 38–41 (2014)
C.A. Randall, Z. Fan, I. Reaney, L.-Q. Chen, S.T. McKinstry, Antiferroelectrics: history, fundamentals, crystal chemistry, crystal structures, size effects, and applications. J. Am. Ceram. Soc. 104, 3775–3810 (2021)
K. Banerjee, S. Asthana, Scaling behavior of different shapes of hysteresis loops and recoverable energy storage density in Na0.5Bi0.5TiO3, K0.5Bi0.5TiO3, and Na0.25K0.25Bi0.5TiO3 ferroelectrics. J. Materiomics. 8, 918–927 (2022)
N. Srivastava, G.J. Weng, A theory of double hysteresis for ferroelectric crystals. J. Appl. Phys. 99, 054103–054111 (2006)
V.U. Rahangdale, D.K. Gala, R.M. Acharya, V.K. Deshpande, Dielectric and ferroelectric properties of Al2O3 containing lead titanate based glass-ceramics, AIP Conf. Proc. 1591 (2014) 705–707
J. Shankar, G.N. Rani, J. Anjaiah, P. Raju, V.K. Deshpande, Study of Microstructure and Dielectric Properties of PbTiO3 based Glass Ceramics, AIP Conference Proceedings 2162 (2019) 020045-6
H. Qiao, C. He, Z. Wang, D. Pang, X. Li, Y. Liu, X. Long, Influence of Mn dopants on the electrical properties of pb(In0.5Nb0.5)O3-PbTiO3 ferroelectric single crystals. RSC Adv. 7, 32607–32612 (2017)
H.-I. Hsiang, K.-Y. Lin, F.-S. Yen, C.-Y. Hwang, Effects of particle size of BaTiO3 powder on the dielectric properties of BaTiO3/polyvinylidene fluoride composites. J. Mater. Sci. 36, 3809–3815 (2001)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All listed authors have participated in the research presented in this paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Khalek, E.K., Mohamed, E.A. & Ismail, Y.A.M. Double hysteresis (P-E) loops in the transparent composite containing BaTiO3 at room temperature. Appl. Phys. A 130, 387 (2024). https://doi.org/10.1007/s00339-024-07495-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07495-7