Abstract
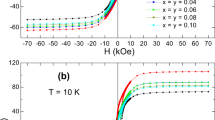
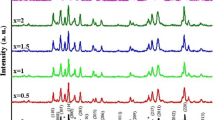
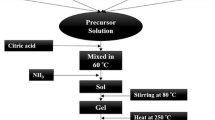
(BaFe12O19)1 − x/(MgO)x nanocomposites, with weight fraction x = 0, 0.1, 0.2, 0.4, 0.8 and 1, have been synthesized by co-precipitation method followed by high-speed ball-milling techniques. Structural, optical, and magnetic properties have been studied. XRD analysis confirmed the formation of the two pure phases BaFe12O19 and MgO, with the formation of a MgFe2O4 as a minor phase in the nanocomposites. Transmission electron microscopy (TEM) followed by high resolution TEM and SAED were used to study the morphology, crystallinity and lattice spacing, respectively. TEM micrographs revealed the co-existence of spherical and nanorod-shaped particles for pure BaFe12O19, and cubic shape for MgO nanoparticles. Raman spectrum for BaFe12O18 showed strong and sharp modes, identifying the formation of barium hexaferrite phase. However, MgO showed two broad peaks attributed to G and D bands. The energy dispersive X-ray and scanning electron microscope were performed for elemental analysis and surface topography, respectively. The real elemental compositions matched well with the starting values. X-ray photoelectron spectroscopy was conducted for the investigation of the elemental compositions and the oxidation states of (Ba2+, Fe2+, Fe3+ Mg2+ and O2−). The magnetic properties like saturation magnetization, remanent magnetization, coercivity and squareness ratio have been determined using the M–H loops. These loops revealed the hard ferromagnetic behavior of pure barium hexaferrite and the mixture of ferromagnetism with diamagnetic behavior at high fields for pure MgO. The saturation and remanent magnetizations decreased with the addition of MgO phase. dM/dH curves showed a weak magnetic coupling between the hard magnetic BaFe12O19 and the soft magnetic MgFe2O4.


















Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
M. Pardavi-Horvath, Microwave applications of soft ferrites. J. Magn. Magn. Mater. 215–216, 171–183 (2000). https://doi.org/10.1016/S0304-8853(00)00106-2
V.G. Harris, Modern Microwave Ferrites. IEEE Trans. Magn. 48(3), 1075–1104 (2012). https://doi.org/10.1109/TMAG.2011.2180732
S.E. Jacobo, C. Domingo-Pascual, R. Rodriguez-Clemente, M.A. Blesa, Synthesis of ultrafine particles of barium ferrite by chemical coprecipitation. J. Mater. Sci. 32(4), 1025–1028 (1997). https://doi.org/10.1023/A:1018582423406
R.C. Pullar, M.D. Taylor, A.K. Bhattacharya, Novel aqueous sol–gel preparation and characterization of barium M ferrite, BaFe12O19 fibres. J. Mater. Sci. 32(2), 349–352 (1997). https://doi.org/10.1023/A:1018593014378
M.A. Almessiere et al., Review on functional bi-component nanocomposites based on hard/soft ferrites: Structural, magnetic, electrical and microwave absorption properties. Nano-Struct. Nano-Obj. 26, 100728 (2021). https://doi.org/10.1016/j.nanoso.2021.100728
Enhancement on the exchange coupling behavior of SrCo0.02Zr0.02Fe11.96O19/MFe2O4 (M=Co, Ni, Cu, Mn and Zn) as hard/soft magnetic nanocomposites. https://en.x-mol.com/paper/article/6015309. Accessed 30 Jan 2023
M. Zareef Khan, H. Abbas, K. Nadeem, A. Iqbal, I.-L. Papst, Concentration dependent exchange coupling in BaFe12O19/NiFe2O4 nanocomposites. J. Alloys Compd. 922, 166105 (2022). https://doi.org/10.1016/j.jallcom.2022.166105
S. Manjura Hoque et al., Exchange-spring mechanism of soft and hard ferrite nanocomposites. Mater. Res. Bull. 48(8), 2871–2877 (2013). https://doi.org/10.1016/j.materresbull.2013.04.009
C. Pahwa, S. Mahadevan, S.B. Narang, P. Sharma, Structural, magnetic and microwave properties of exchange coupled and non-exchange coupled BaFe12O19/NiFe2O4 nanocomposites. J. Alloy. Compd. 725, 1175–1181 (2017). https://doi.org/10.1016/j.jallcom.2017.07.220
H. Yang, M. Liu, Y. Lin, Y. Yang, Simultaneous enhancements of remanence and (BH)max in BaFe12O19/CoFe2O4 nanocomposite powders. J. Alloy. Compd. 631, 335–339 (2015). https://doi.org/10.1016/j.jallcom.2015.01.012
F. Li, Q. Yang, D.G. Evans, X. Duan, Synthesis of magnetic nanocomposite MgO/MgFe2O4 from Mg-Fe layered double hydroxides precursors. J. Mater. Sci. 40(8), 1917–1922 (2005). https://doi.org/10.1007/s10853-005-1211-9
K. Ali, J. Iqbal, T. Jana, N. Ahmad, I. Ahmad, D. Wan, Enhancement of microwaves absorption properties of CuFe2O4 magnetic nanoparticles embedded in MgO matrix. J. Alloy. Compd. 696, 711–717 (2017). https://doi.org/10.1016/j.jallcom.2016.10.220
H. Helmiyati, G.H. Abbas, Y. Budiman, S. Ramadhani, Synthesis of MgFe2O4-MgO nanocomposite: influence of MgO on the catalytic activity of magnesium ferrite in biodiesel production. Rasayan J. Chem. J. Chem. 13, 298–305 (2020). https://doi.org/10.31788/RJC.2020.1315497
R.S. Yadav et al., Magnetic properties of Co1−xZnxFe2O4 spinel ferrite nanoparticles synthesized by starch-assisted sol–gel autocombustion method and its ball milling. J. Magn. Magn. Mater. 378, 190–199 (2015). https://doi.org/10.1016/j.jmmm.2014.11.027
A. Amri, Z.T. Jiang, T. Pryor, C.-Y. Yin, S. Djordjevic, Developments in the synthesis of flat plate solar selective absorber materials via sol–gel methods: a review. Renew. Sustain. Energy Rev. 36, 316–328 (2014). https://doi.org/10.1016/j.rser.2014.04.062
A. Hajalilou, S.A. Mazlan, K. Shameli, A comparative study of different concentrations of pure Zn powder effects on synthesis, structure, magnetic and microwave-absorbing properties in mechanically-alloyed Ni–Zn ferrite. J. Phys. Chem. Solids 96–97, 49–59 (2016). https://doi.org/10.1016/j.jpcs.2016.05.001
R. Yensano, S. Phokha, Effect of pH on single phase BaFe12O19 nanoparticles and their improved magnetic properties. J. Mater. Sci. Mater. Electron. 31(14), 11764–11773 (2020). https://doi.org/10.1007/s10854-020-03728-6
W. Wang, X. Qiao, J. Chen, F. Tan, H. Li, Influence of titanium doping on the structure and morphology of MgO prepared by coprecipitation method. Mater. Char. 60(8), 858–862 (2009). https://doi.org/10.1016/j.matchar.2009.02.002
K. Habanjar, H. Shehabi, A.M. Abdallah, R. Awad, Effect of calcination temperature and cobalt addition on structural, optical and magnetic properties of barium hexaferrite BaFe12O19 nanoparticles. Appl. Phys. A 126(6), 402 (2020). https://doi.org/10.1007/s00339-020-03497-3
K. Kumar, A. Loganathan, Structural, electrical and magnetic properties of large ionic size Sr2+ ions substituted Mg-Ferrite nanoparticles. Mater. Chem. Phys. 214, 229–238 (2018). https://doi.org/10.1016/j.matchemphys.2018.04.067
A. Azhari, S.M. Sharif, F. Golestanifard, A. Saberi, Phase evolution in Fe2O3/MgO nanocomposite prepared via a simple precipitation method. Mater. Chem. Phys. 124(1), 658–663 (2010). https://doi.org/10.1016/j.matchemphys.2010.07.030
S. Kundu, B. Satpati, T. Kar, S.K. Pradhan, Microstructure characterization of hydrothermally synthesized PANI/V2O5·nH2O heterojunction photocatalyst for visible light induced photodegradation of organic pollutants and non-absorbing colorless molecules. J. Hazard. Mater. 339, 161–173 (2017). https://doi.org/10.1016/j.jhazmat.2017.06.034
M. Yassine, N. El Ghouch, A.M. Abdallah, K. Habanjar, R. Awad, Structure and magnetic investigation of hard/soft Ba0.5Sr0.5Fe12O19/x(Ni0.5Zn0.5)Fe2O4 nanocomposite. J. Alloy. Compd. 907, 164501 (2022). https://doi.org/10.1016/j.jallcom.2022.164501
R.S. Alam, M. Moradi, M. Rostami, H. Nikmanesh, R. Moayedi, Y. Bai, Structural, magnetic and microwave absorption properties of doped Ba-hexaferrite nanoparticles synthesized by co-precipitation method. J. Magn. Magn. Mater. 381, 1–9 (2015). https://doi.org/10.1016/j.jmmm.2014.12.059
W. Qin, T. Nagase, Y. Umakoshi, J.A. Szpunar, Relationship between microstrain and lattice parameter change in nanocrystalline materials. Philos. Mag. Lett. 88(3), 169–179 (2008). https://doi.org/10.1080/09500830701840155
T. Kaur, S. Kumar, B.H. Bhat, A.K. Srivastava, Enhancement in physical properties of barium hexaferrite with substitution. J. Mater. Res. 30(18), 2753–2762 (2015). https://doi.org/10.1557/jmr.2015.244
H. Shang, J. Wang, Q. Liu, Synthesis and characterization of nanocrystalline BaFe12O19 obtained by using glucose as a fuel. Mater. Sci. Eng. A 456(1), 130–132 (2007). https://doi.org/10.1016/j.msea.2006.12.011
S. Vadivelan, N. Victor Jaya, Investigation of magnetic and structural properties of copper substituted barium ferrite powder particles via co-precipitation method. Res. Phys. 6, 843–850 (2016). https://doi.org/10.1016/j.rinp.2016.07.013
G. Kandregula, Structural properties of MgO nanoparticles: synthesized by co-precipitation technique. Int. J. Sci. Res. (IJSR) 3, 43–46 (2014)
J. Wang, Y. Wu, Y. Zhu, P. Wang, Formation of rod-shaped BaFe12O19 nanoparticles with well magnetic properties. Mater. Lett. 61(7), 1522–1525 (2007). https://doi.org/10.1016/j.matlet.2006.07.183
I. Altman, I. Agranovski, M. Choi, On nanoparticle surface growth: MgO nanoparticle formation during a Mg particle combustion. Appl. Phys. Lett. 84, 5130–5132 (2004). https://doi.org/10.1063/1.1764937
I.Y. Younis, S.S. El-Hawary, O.A. Eldahshan, M.M. Abdel-Aziz, Z.Y. Ali, Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 11, 16868 (2021). https://doi.org/10.1038/s41598-021-96377-6
V. Šepelák et al., The mechanically induced structural disorder in barium hexaferrite, BaFe12O19, and its impact on magnetism. Faraday Discuss. 170, 121–135 (2014). https://doi.org/10.1039/C3FD00137G
S. Liu, P. Gao, H. Zou, B. Qin, J. He, L. Deng, Large magnetoelectric effect in BaFe12O19-(Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 particulate composite. Adv. Powder Mater. 1(3), 100022 (2022). https://doi.org/10.1016/j.apmate.2021.12.001
R.S. Azis et al., Effect of ratio in ammonium nitrate on the structural, microstructural, magnetic, and AC conductivity properties of BaFe12O19. Materials (Basel) 11(11), 2190 (2018). https://doi.org/10.3390/ma11112190
S. Maensiri, M. Sangmanee, A. Wiengmoon, Magnesium ferrite (MgFe2O4) nanostructures fabricated by electrospinning. Nanoscale Res. Lett. (2009). https://doi.org/10.1007/s11671-008-9229-y
P. Sharma, A. Kumar, A. Dube, Q. Li, D. Varshney, Structural and dielectric properties of La and Ni-doped M-type BaFe12O19 ceramics. AIP Conf. Proc. 1731(1), 140010 (2016). https://doi.org/10.1063/1.4948176
J. Kreisel, G. Lucazeau, H. Vincent, Raman spectra and vibrational analysis of BaFe12O19 hexagonal ferrite. J. Solid State Chem. 137(1), 127–137 (1998). https://doi.org/10.1006/jssc.1997.7737
W.Y. Zhao, P. Wei, X.Y. Wu, W. Wang, Q.J. Zhang, Lattice vibration characterization and magnetic properties of M-type barium hexaferrite with excessive iron. J. Appl. Phys. 103(6), 063902 (2008). https://doi.org/10.1063/1.2884533
R. Pandey, L. Kumar Pradhan, S. Kumari, M. Kumar Manglam, S. Kumar, M. Kar, Surface magnetic interactions between Bi0.85La0.15FeO3 and BaFe12O19 nanomaterials in (1 − x)Bi0.85La0.15FeO3-(x)BaFe12O19 nanocomposites. J. Magnet. Magnet. Mater. 508, 166862 (2020). https://doi.org/10.1016/j.jmmm.2020.166862
S. Mallesh, D. Prabu, V. Srinivas, Thermal stability and magnetic properties of MgFe2O4@ZnO nanoparticles. AIP Adv. 7(5), 056103 (2017). https://doi.org/10.1063/1.4975355
A. Weibel, D. Mesguich, G. Chevallier, E. Flahaut, C. Laurent, Fast and easy preparation of few-layered-graphene/magnesia powders for strong, hard and electrically conducting composites. Carbon 136, 270–279 (2018). https://doi.org/10.1016/j.carbon.2018.04.085
T. Athar, A. Deshmukh, W. Ahmed, Synthesis of MgO nanopowder via non aqueous sol-gel method. Adv. Sci. Lett. 5, 1–3 (2012). https://doi.org/10.1166/asl.2012.2190
P. Dobrosz, S.J. Bull, S.H. Olsen, A.G. O’Neill, “Measurement of the residual macro and microstrain in strained Si/SiGe using Raman spectroscopy. MRS Online Proc. Lib. (OPL) 809, B3.4 (2004). https://doi.org/10.1557/PROC-809-B3.4
R.J. Angel, M. Murri, B. Mihailova, M. Alvaro, Stress, strain and Raman shifts. Z. Kristallogr. Crystall. Mater. 234(2), 129–140 (2019). https://doi.org/10.1515/zkri-2018-2112
F. Naaz, H. Dubey, C. Kumari, P. Lahiri, Structural and magnetic properties of MgFe2O4 nanopowder synthesized via co-precipitation route. SN Appl. Sci. (2020). https://doi.org/10.1007/s42452-020-2611-9
P.J. Burke, Z. Bayindir, G.J. Kipouros, X-ray photoelectron spectroscopy (XPS) investigation of the surface film on magnesium powders. Appl. Spectrosc. 66(5), 510–518 (2012). https://doi.org/10.1366/11-06372
B.C. Brightlin, S. Balamurugan, The effect of post annealing treatment on the citrate sol–gel derived nanocrystalline BaFe12O19 powder: structural, morphological, optical and magnetic properties. Appl. Nanosci. 6(8), 1199–1210 (2016). https://doi.org/10.1007/s13204-016-0531-1
F. Mirza, H. Makwana, Synthesis and characterization of magnesium oxide (MgO) nanoparticles by co-precipitation method, 8(6), 7
A. Rodrigues, S. Bauer, T. Baumbach, Effect of post-annealing on the chemical state and crystalline structure of PLD Ba0.5Sr0.5TiO3 films analyzed by combined synchrotron X-ray diffraction and X-ray photoelectron spectroscopy. Ceram. Int. 44(13), 16017–16024 (2018). https://doi.org/10.1016/j.ceramint.2018.06.038
F. Alema, K. Pokhodnya, Dielectric properties of BaMg1∕3Nb2∕3O3 doped Ba0.45Sr0.55TiO3 thin films for tunable microwave applications. J. Adv. Dielect. 5(4), 1550030 (2015). https://doi.org/10.1142/S2010135X15500307
R. Perez-Gonzalez et al., Highly efficient flexible CNT based supercapacitors fabricated with magnetic BaFe12O19 nanoparticles and biodegradable components. J. Phys. Chem. Solids 155, 110115 (2021). https://doi.org/10.1016/j.jpcs.2021.110115
X. Zhang, Y. Zhang, Z. Yue, J. Zhang, Influences of sintering atmosphere on the magnetic and electrical properties of barium hexaferrites. AIP Adv. 9(8), 085129 (2019). https://doi.org/10.1063/1.5111422
Q. Li et al., Vacancy-engineered Gd3+-substituted yttrium iron garnet with narrow ferrimagnetic linewidth and high Curie temperature. J. Alloy. Compd. 935, 168169 (2023). https://doi.org/10.1016/j.jallcom.2022.168169
C. Liu, Y. Zhang, J. Jia, Q. Sui, N. Ma, P. Du, Multi-susceptibile single-phased ceramics with both considerable magnetic and dielectric properties by selectively doping. Sci. Rep. (2015). https://doi.org/10.1038/srep09498
H. Cui, X. Wu, Y. Chen, J. Zhang, R.I. Boughton, Influence of copper doping on chlorine adsorption and antibacterial behavior of MgO prepared by co-precipitation method. Mater. Res. Bull. 61, 511–518 (2015). https://doi.org/10.1016/j.materresbull.2014.10.067
K.S. Sánchez-Zambrano et al., XPS study on calcining mixtures of brucite with Titania. Materials (Basel) 15(9), 3117 (2022). https://doi.org/10.3390/ma15093117
J. Lu, X. Wei, Y. Chang, S. Tian, Y. Xiong, Role of Mg in mesoporous MgFe2O4 for efficient catalytic ozonation of Acid Orange II. J. Chem. Technol. Biotechnol. 91(4), 985–993 (2016). https://doi.org/10.1002/jctb.4667
A.A. Bezlepkin, S.P. Kuntsevich, Domain structure of hexaferrite BaFe12O19 near the Curie temperature. Phys. Solid State 62(7), 1179–1182 (2020). https://doi.org/10.1134/S1063783420070045
K. Tanwar, D.S. Gyan, P. Gupta, S. Pandey, D. Kumar, Investigation of crystal structure, microstructure and low temperature magnetic behavior of Ce4+ and Zn2+ co-doped barium hexaferrites (BaFe12O19). RSC Adv. 8(35), 19600–19609 (2018). https://doi.org/10.1039/C8RA02455C
V. Babu, P. Padaikathan, Structure and hard magnetic properties of barium hexaferrite with and without La2O3 prepared by ball milling. J. Magn. Magn. Mater. 241(1), 85–88 (2002). https://doi.org/10.1016/S0304-8853(01)00811-3
S.N. Attyabi, S.A. Seyyed Ebrahimi, Z. Lalegani, B. Hamawandi, Reverse magnetization behavior investigation of Mn-Al-C-(α-Fe) nanocomposite alloys with different α-Fe content using first-order reversal curves analysis. Nanomaterials (2022). https://doi.org/10.3390/nano12193303
M.K. Manglam, J. Mallick, S. Kumari, R. Pandey, M. Kar, Crystal structure and magnetic properties study on barium hexaferrite (BHF) and cobalt zinc ferrite (CZF) in composites. Solid State Sci. 113, 106529 (2021). https://doi.org/10.1016/j.solidstatesciences.2020.106529
N.A. Algarou et al., Magnetic and microwave properties of SrFe12O19/MCe0.04Fe1.96O4 (M = Cu, Ni, Mn, Co and Zn) hard/soft nanocomposites. J. Market. Res. 9(3), 5858–5870 (2020). https://doi.org/10.1016/j.jmrt.2020.03.113
Y. Lin, P. Kang, H. Yang, M. Liu, Preparation and characterization of BaFe12O19/Y3Fe5O12 composites. J. Alloy. Compd. 641, 223–227 (2015). https://doi.org/10.1016/j.jallcom.2015.03.265
X. Shen, F. Song, J. Xiang, M. Liu, Y. Zhu, Y. Wang, Shape anisotropy, exchange-coupling interaction and microwave absorption of hard/soft nanocomposite ferrite microfibers. J. Am. Ceram. Soc. 95(12), 3863–3870 (2012). https://doi.org/10.1111/j.1551-2916.2012.05375.x
M.K. Manglam, S. Kumari, S. Guha, S. Datta, M. Kar, Study of magnetic interaction between hard and soft magnetic ferrite in the nanocomposite. AIP Conf. Proc. 2220(1), 110020 (2020). https://doi.org/10.1063/5.0001220
S. Datta, M.K. Manglam, S.K. Panda, A. Shukla, M. Kar, Investigation of crystal structure and magnetic properties in magnetic composite of soft magnetic alloy and hard magnetic ferrite. Phys. B 653, 414675 (2023). https://doi.org/10.1016/j.physb.2023.414675
M.S. Seehra, S. Suri, V. Singh, Effects of Cu doping on the magnetism of CeO2 nanoparticles. J. Appl. Phys. 111(7), 07B516 (2012). https://doi.org/10.1063/1.3676223
M.A. Almessiere, Y. Slimani, A. Baykal, Structural and magnetic properties of Ce-doped strontium hexaferrite. Ceram. Int. 44(8), 9000–9008 (2018). https://doi.org/10.1016/j.ceramint.2018.02.101
V.N. Dhage, M.L. Mane, M.K. Babrekar, C.M. Kale, K.M. Jadhav, Influence of chromium substitution on structural and magnetic properties of BaFe12O19 powder prepared by sol–gel auto combustion method. J. Alloy. Compd. 509(12), 4394–4398 (2011). https://doi.org/10.1016/j.jallcom.2011.01.040
Z. Mosleh, P. Kameli, M. Ranjbar, H. Salamati, Effect of annealing temperature on structural and magnetic properties of BaFe12O19 hexaferrite nanoparticles. Ceram. Int. 40(5), 7279–7284 (2014). https://doi.org/10.1016/j.ceramint.2013.12.068
P. Xu, X. Han, M. Wang, Synthesis and magnetic properties of BaFe12O19 hexaferrite nanoparticles by a reverse microemulsion technique. J. Phys. Chem. C 111(16), 5866–5870 (2007). https://doi.org/10.1021/jp068955c
N. Cordente, M. Respaud, F. Senocq, M.-J. Casanove, C. Amiens, B. Chaudret, Synthesis and magnetic properties of nickel nanorods. Nano Lett. 1(10), 565–568 (2001). https://doi.org/10.1021/nl0100522
S. Phokha, J. Klinkaewnarong, S. Hunpratub, K. Boonserm, E. Swatsitang, S. Maensiri, Ferromagnetism in Fe-doped MgO nanoparticles. J. Mater. Sci. Mater. Electron. 27(1), 33–39 (2016). https://doi.org/10.1007/s10854-015-3713-9
S. Azzaza et al., Structural, optical and magnetic characterizations of Mn-doped MgO nanoparticles. Mater. Chem. Phys. 143(3), 1500–1507 (2014). https://doi.org/10.1016/j.matchemphys.2013.12.006
A. Almontasser, A. Parveen, Probing the effect of Ni, Co and Fe doping concentrations on the antibacterial behaviors of MgO nanoparticles. Sci. Rep. (2022). https://doi.org/10.1038/s41598-022-12081-z
N. Pathak et al., Defect induced ferromagnetism in MgO and its exceptional enhancement upon thermal annealing: a case of transformation of various defect states. Phys. Chem. Chem. Phys. 19(19), 11975–11989 (2017). https://doi.org/10.1039/C7CP01776F
N. Kumar, D. Sanyal, A. Sundaresan, Defect induced ferromagnetism in MgO nanoparticles studied by optical and positron annihilation spectroscopy. Chem. Phys. Lett. 477, 360–364 (2009). https://doi.org/10.1016/j.cplett.2009.07.037
F. Gao et al., First-principles study of magnetism driven by intrinsic defects in MgO. Solid State Commun. 149(21), 855–858 (2009). https://doi.org/10.1016/j.ssc.2009.03.010
R.D. Widodo, A. Manaf, Physical characteristics and magnetic properties of BaFe12O19/SrTiO3 based composites derived from mechanical alloying. AIP Conf. Proc. 1725(1), 020098 (2016). https://doi.org/10.1063/1.4945552
L. Cao, Z. Wang, Z. Ye, Y. Zhang, L. Zhao, Y. Zeng, Interface exchange coupling induced enhancements in coercivity and maximal magnetic energy product of BaFe12O19/Co3O4 nanocomposites. J. Alloy. Compd. 715, 199–205 (2017). https://doi.org/10.1016/j.jallcom.2017.04.284
J. Sung Lee, J. Myung Cha, H. Young Yoon, J.-K. Lee, Y. Keun Kim, Magnetic multi-granule nanoclusters: a model system that exhibits universal size effect of magnetic coercivity. Sci. Rep. (2015). https://doi.org/10.1038/srep12135
E. Suharyadi, A. Hermawan, D.L. Puspitarum, Crystal structure and magnetic properties of magnesium ferrite (MgFe2O4) nanoparticles synthesized by coprecipitation method. J. Phys. Conf. Ser. 1091(1), 012003 (2018). https://doi.org/10.1088/1742-6596/1091/1/012003
N. Alghamdi et al., Structural, magnetic and toxicity studies of ferrite particles employed as contrast agents for magnetic resonance imaging thermometry. J. Magn. Magn. Mater. 497, 165981 (2020). https://doi.org/10.1016/j.jmmm.2019.165981
Y. Yang, D. Huang, F. Wang, J. Shao, An investigation on microstructural, spectral and magnetic properties of Pr–Cu double-substituted M-type Ba–Sr hexaferrites. Chin. J. Phys. 57, 250–260 (2019). https://doi.org/10.1016/j.cjph.2018.11.012
F. Fattouh et al., Structural and magnetic properties of hard-soft BaFe12O19/(Zn0.5Co0.5)Fe2O4 ferrites. J. Phys. Condens. Matter (2021). https://doi.org/10.1088/1361-648X/abf478
Acknowledgements
Authors declare their genuine gratitude and appreciation to Faculty of Science at Alexandria University in Egypt, Central Metallurgical Research & Development Institute (Helwan, Egypt) and Advanced Materials Science Lab at BAU (Debbieh, Lebanon).
Author information
Authors and Affiliations
Contributions
MS performed the measurements, processed the experimental data, and drafted the manuscript. Prof RA and Dr. KH were involved in planning and supervised the work. Prof RA and Dr. KH discussed the results and commented on the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharrouf, M., Awad, R. & Habanjar, K. Investigation of the structural, morphological and magnetic properties of barium hexaferrite added with magnesium oxide nanoparticles. Appl. Phys. A 129, 807 (2023). https://doi.org/10.1007/s00339-023-07079-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07079-x