Abstract
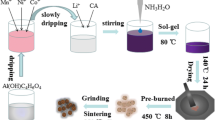
A simple solvothermal route was successfully implemented for fabrication of Li-rich Mn-based layered cathode Li1.15Ni0.15Co0.15Mn0.7M0.01O2 (LMLC) materials through incorporation of different trivalent cations (M3+: Al3+, Cr3+ or Fe3+). The structural properties of mixed solid solution of rhombohedral LiNiO2 phase and monoclinic Li2MnO3 phases were checked by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). LMLC-Fe sample shows the highest coercivity Hc value of about 198.1 Oe, and the lowest dielectric constant εr about 74. LMLC-Cr sample exhibits the lowest activation energy (Ea = 0.163 eV) for electric conduction as a result of the smallest cell volume and particle size. LMLC-Al electrode material delivers the highest initial charge and discharge capacity of about 140 and 104 mAh/g at 0.1 C, respectively maintaining around 70% of the initial capacity at 0.1C. Both LMLC-Al and LMLC-Cr samples show better high rate capabilities than that of LMLC-Fe sample.










Similar content being viewed by others
References
A.M. Gaikwad, B.V. Khau, G. Davies, B. Hertzberg, D.A. Steingart, A.C. Arias, A high areal capacity flexible lithium-ion battery with a strain-compliant design. Adv. Energy Mater. 5, 1401389 (2015). https://doi.org/10.1002/aenm.201401389
M.S. Whittingham, Introduction: batteries. Chem. Rev. 114, 11413–11413 (2014). https://doi.org/10.1021/cr500639y
Z. Lu, D.D. Macneil, J.R. Dahn, Layered cathode materials Li[Nix(2/3–x/3)]O2 for lithium-ion batteries. Electrochem. Solid-State Lett. 34, 191 (2001). https://doi.org/10.1149/1.1407994
M.M. Thackeray, S.H. Kang, C.S. Johnson, J.T. Vaughey, S.A. Hackney, Comments on the structural complexity of lithium-rich Li1+ xM1− xO2 electrodes (M= Mn, Ni, Co) for lithium batteries. Electrochem. Commun. 8, 1531–1538 (2006). https://doi.org/10.1016/j.elecom.2006.06.030
X.J. Guo, Y.X. Li, M. Zheng, J.M. Zheng, J. Li, Z.L. Gong, Y. Yang, Structural and electrochemical characterization of xLi[Li]1/3Mn2/3O2-(1-x)Li[Ni1/3Mn1/3Co1/3]O2 (0<x<0.9) as cathode materials for Li ion batteries. J Power Sources 184, 414–419 (2008). https://doi.org/10.1016/j.jpowsour.2008.04.013
H. Yu, H. Kim, Y. Wang, P. He, D. Asakura, Y. Nakamura, H. Zhou, High-energy composite layered manganese-rich cathode materials via controlling Li2MnO3 phase activation for lithium-ion batteries. Phys. Chem. Chem. Phys. 14, 6584–6595 (2012). https://doi.org/10.1039/C2CP40745K
H. Pan, T. Zhu, S. Zhang, Y. Jiang, J. Chen, M. Gao, Y. Liu, Li- and Mn-rich layered oxide cathode materials for lithium-ion batteries: a review from fundamentals to research progress and applications. Mol. Syst. Des. Eng. 3, 748–803 (2018). https://doi.org/10.1039/c8me00025e
A. Manthiram, J.C. Knight, S.-T. Myung, S.-M. Oh, Y.-K. Sun, M. Gu, I. Belharouak, Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv. Energy Mater. 6, 1501010 (2016). https://doi.org/10.1002/aenm.201501010
X. Yang, X. Wang, Q. Wei, H. Shu, L. Liu, S. Yang, B. Hu, Y. Song, G. Zou, L. Hu, L. Yi, Synthesis and characterization of a Li-rich layered cathode material Li1.15[(Mn1/3Ni1/3Co1/3)0.5(Ni1/4Mn3/4)0.5]0.85O2 with spherical core–shell structure. J. Mater. Chem. 22, 19666–19672 (2012). https://doi.org/10.1039/C2JM34259F
H.J. Yu, H.S. Zhou, High-energy cathode materials (Li2MnO3–LiMO2) for lithium-ion batteries. J. Phys. Chem. Lett. 4, 1268–1280 (2013). https://doi.org/10.1021/jz400032v
X. Dong, Y.L. Xu, S. Yan, S.C. Mao, L.L. Xiong, X.F. Sun, Towards low-cost, high energy density Li2MnO3 cathode materials. J. Mater. Chem. A 3, 670–679 (2015). https://doi.org/10.1039/C4TA02924K
J.R. Croy, D. Kim, M. Balasubramanian, K. Gallagher, S.H. Kang, M.M. Thackeray, Countering the voltage decay in high capacity xLi2MnO3-(1–x)LiMO2 electrodes (M: Mn Ni, Co) for Li+-ion batteries. J. Electrochem. Soc. 159, A781–A790 (2012). https://doi.org/10.1149/2.080206jes
S. Yang, G. Huang, S. Hu, X. Hou, Y. Huang, M. Yue, G. Lei, Improved electrochemical performance of the Li1.2Ni0.13Co0.13Mn0.54O2 wired by CNT networks for Li-ion batteries. Mater. Lett. 118, 8–11 (2014). https://doi.org/10.1016/j.matlet.2013.11.071
J.R. Croy, M. Balasubramanian, K.G. Gallagher, A.K. Burrell, Review of the U.S. Department of Energy’s “deep dive” to understand voltage fade in Li- and Mn-rich cathodes. Acc. Chem. Res. 48, 2813–2821 (2015). https://doi.org/10.1021/acs.accounts.5b00277
A. Kumar, R. Nazzario, L. Torres-Castro, A. Pena-Duarte, M.S. Tomar, Electrochemical properties of MgO-coated 0.5 Li2MnO3–0.5 LiNi0.5Mn0.5O2 composite cathode material for lithium ion battery. Int. J. Hydrog. Energy 40, 4931–4935 (2015)
L. Zhong, H. Jian-He, H. Gang, L. Lu, Effect of FePO4 coating on performance of Li1.2Mn0.54Ni0.13Co0.13O2 as cathode material for Li-ion battery. J. Inorg. Mater. 30, 129–134 (2015)
S. Yamamoto, H. Noguchi, W.W. Zhao, Improvement of cycling performance in Ti substituted 0.5Li2MnO3–0.5LiNi0.5Mn0.5O2 through suppressing metal dissolution. J. Power Sources 278, 76–86 (2015). https://doi.org/10.1016/j.jpowsour.2014.12.038
H. Chen, Q. Hu, Z. Huang, Z. He, Z. Wang, H. Guo, X. Li, Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram. Inter. 42, 263–269 (2016). https://doi.org/10.1016/j.ceramint.2015.08.104
Y. Zang, C. Ding, X. Wang, Z. Wen, C. Chen, Molybdenum-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with high specific capacity and improved rate performance. Electrochem. Acta 168, 234–239 (2015). https://doi.org/10.1016/j.electacta.2015.03.223
Z. He, Z. Wang, H. Guo, X. Li, P. Yue, J. Wang, X. Xiong, Synthesis and electrochemical performance of xLi2MnO3·(1–x)LiMn0.5Ni0.4Co0.1O2 for lithium ion battery. Powder Technol. 235, 158–162 (2013). https://doi.org/10.1016/j.powtec.2012.09.020
H. Xu, S. Deng, G. Chen, Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by Mg doping for lithium ion battery cathode material. J. Mater. Chem. 2, 15015–15021 (2014). https://doi.org/10.1039/C4TA01790K
H. Li, H. Guo, Z. Wang, J. Wang, X. Li, N. Chen, W. Gui, Improving rate capability and decelerating voltage decay of Li-rich layered oxide cathodes by chromium doping. Inter. Journal Hydr. Ener. 43, 11109–11119 (2018). https://doi.org/10.1016/j.ijhydene.2018.04.203
F. Nobili, F. Croce, R. Tossici, I. Meschini, P. Reale, R. Marassi, Sol–gel synthesis and electrochemical characterization of Mg-/Zr-doped LiCoO2 cathodes for Li-ion batteries. J. Power Sources 197, 276–284 (2012). https://doi.org/10.1016/j.jpowsour.2011.09.053
L. Croguennec, J. Bains, J. Bréger, C. Tessier, P. Biensan, S. Levasseur, C. Delmas, Effect of aluminum substitution on the structure, electrochemical performance and thermal stability of Li1+x(Ni0.40Mn0.40Co0.20−zAlz)1−xO2. J. Electrochem. Soc. 158, A664–A670 (2011). https://doi.org/10.1149/1.3571479
C. Lu, S. Yang, H. Wu, Y. Zhang, X. Yang, T. Liang, Enhanced electrochemical performance of Li-rich Li1.2Mn0.52Co0.08Ni0.2O2 cathode materials for Li-ion batteries by vanadium doping. Electrochem. Acta 209, 448–455 (2016). https://doi.org/10.1016/j.electacta.2016.05.119
X. Feng, Y. Gao, L. Ben, Z. Yang, Z. Wang, L. Chen, Enhanced electrochemical performance of Ti-doped Li1.2Mn0.54Co0.13Ni0.13O2 for lithium-ion batteries. J. Power Sources 317, 74–80 (2016). https://doi.org/10.1016/j.jpowsour.2016.03.101
Y. Zhao, G. Sun, R. Wu, Synthesis of nanosized Fe-Mn based Li-rich cathode materials for lithium-ion battery via a simple method. Electroch. Acta 96, 291–297 (2013). https://doi.org/10.1016/j.electacta.2013.01.059
Q.-Q. Qiao, L. Qin, G.-R. Li, Y.-L. Wang, X.-P. Gao, Sn-stabilized Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as a cathode for advanced lithium-ion batteries. J. Mater. Chem. A 3, 17627–17634 (2015). https://doi.org/10.1039/C5TA03415A
C.-C. Wang, Y.-C. Lin, P.-H. Chou, Mitigation of layer to spinel conversion of a lithium-rich layered oxide cathode by substitution of Al in a lithium ion battery. RSC Adv. 5, 68919–68928 (2015). https://doi.org/10.1039/C5RA11665A
P.K. Nayak, J. Grinblat, M. Levi, E. Levi, S. Kim, J.W. Choi, D. Aurbach, Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv. Energy Mater. (2016). https://doi.org/10.1002/aenm.201502398
P.K. Nayak, J. Grinblat, E. Levi, M. Levi, B. Markovsky, D. Aurbach, Understanding the influence of Mg doping for the stabilization of capacity and higher discharge voltage of Li- and Mn-rich cathodes for Li- ion batteries. Phys. Chem. Chem. Phys. 19, 6142–6152 (2017). https://doi.org/10.1039/C6CP07383B
Y.-J. Kang, J.-H. Kim, S.-W. Lee, Y.-K. Sun, The effect of Al(OH)3 coating on the Li[Li0.2Ni0.2Mn0.6]O2 cathode material for lithium secondary battery. Electroch. Acta 50, 4784–4791 (2005). https://doi.org/10.1016/j.electacta.2005.02.032
T. Zhao, S. Chen, L. Li, X. Zhang, R. Chen, I. Belharouak, F. Wu, K, , Amine, Synthesis, characterization, and electrochemistry of cathode material Li[Li0.2Co0.13Ni0.13Mn0.54]O2 using organic chelating agents for lithium-ion batteries. J. Power Sources 228, 206–213 (2013). https://doi.org/10.1016/j.jpowsour.2012.11.099
F. Fu, Y.Y. Huang, P. Wu, Y.K. Bu, Y.B. Wang, J.N. Yao, Controlled synthesis of lithium-rich layered Li1.2Mn0.56Ni0.12Co0.12O2 oxide with tunable morphology and structure as cathode material for lithium-ion batteries by solvo/hydrothermal methods. J. Alloys Compd. 618, 673–678 (2015). https://doi.org/10.1016/j.jallcom.2014.08.191
E. Markevich, G. Salitra, Y. Talyosef, U.-H. Kim, H.-H. Ryu, Y.-K. Sun, D. Aurbach, High performance LiNiO2 cathodes with practical loading cycled with Li metal anodes in fluoroethylene carbonate based electrolyte solution. ACS Appl. Energy Mater. 1, 2600–2607 (2018). https://doi.org/10.1021/acsaem.8b00304
A. Boulineau, L. Croguennec, C. Delmas, F. Weill, Structure of Li2MnO3 with different degrees of defects. Solid State Ionics 180, 1652–1659 (2010). https://doi.org/10.1016/j.ssi.2009.10.020
S. Alagar, C.-C. Yang, C. Karuppiah, R. Madhuvilakku, S. Piraman, Temperature-controlled synthesis of Li- and Mn-Rich Li1.2Mn0.54Ni0.13Co0.13O2 hollow nano/sub-microsphere electrodes for high-performance lithium-ion battery. ACS Omega 4(23), 20285–20296 (2019). https://doi.org/10.1021/acsomega.9b02766
B. Seteni, N. Rapulenyane, J.C. Ngila, H. Luo, Structural and electrochemical behavior of Li1.2Mn0.54Ni0.13Co0.13-xAlxO2 (x = 0.05) positive electrode material for lithium ion battery. Mater Today Proc 5, 10479–10487 (2018). https://doi.org/10.1016/j.matpr.2017.12.379
S. Rajarathinam, S. Mitra, R.K. Petla, Li2MnO3 rich-LiMn0.33Co0.33Ni0.33O2 integrated nano-composites as high energy density lithium-ion battery cathode materials. Electrochem. Acta 108, 135–144 (2013). https://doi.org/10.1016/j.electacta.2013.06.102
D. Mohanty, A.S. Sefat, S. Kalnaus, J. Li, R.A. Meisner, E.A. Payzant, D.P. Abraham, D.L. Wood, C. Daniel, Investigating phase transformation in the Li1.2Co0.1Mn0.55Ni0.15O2 lithium-ion battery cathode during high-voltage hold (4.5 V) via magnetic, X-ray diffraction and electron microscopy studies. J. Mater. Chem. A 1, 6249–6261 (2013). https://doi.org/10.1039/C3TA10304H
Q. Xia, D.G. Ivey, X. Zhao, M. Xu, W. Wei, Z. Ding, J. Liu, L. Chen, A Li-rich layered@spinel@carbon heterostructured cathode material for high capacity and high rate lithium-ion batteries fabricated via an in situ synchronous carbonization-reduction method. J. Mater. Chem. A 3, 3995–4003 (2015). https://doi.org/10.1039/C4TA05848H
Y. Tian, M. Chen, S. Xue, Y. Cai, Q. Huang, X. Liu, W. Li, Template-determined microstructure and electrochemical performances of li-rich layered metal oxide cathode. J. Power Sources 401, 343–353 (2018). https://doi.org/10.1016/j.jpowsour.2018.09.010
K. Amine, H. Tukamoto, H. Yasuda, Y. Fujita, A new three-volt spinel Li1+xMn1.5Ni0.5O4 for secondary lithium batteries. J. Electrochem. Soc. 143, 1607–1613 (1996). https://doi.org/10.1149/1.1836686
K.M. Shaju, R.G.V. Subbu, B.V.R. Chowdari, Performance of layered LiNi1/3Co1/3Mn1/3O2 as cathode for Li-ion batteries. Electrochim. Acta 48, 145–151 (2002). https://doi.org/10.1016/S0013-4686(02)00593-5
J. Huang, X. Fang, Y. Wu, L. Zhou, Y. Wang, Y. Jin, W. Dang, L. Wu, Z. Rong, X. Chen, X. Tang, Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 by surface modification with lithium-active MoO3. J. Electroanaly. Chem. 823, 359–367 (2018)
Y. Zhao, M. Xia, X. Hu, Z. Zhao, Y. Wang, Z. Lv, Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.8O2 Li-rich cathode materials. Electrochim. Acta 174, 1167–1174 (2015). https://doi.org/10.1016/j.electacta.2015.05.068
Z. Chen, J. Wang, D.L. Chao, T. Baikie, L. Bai, S. Chen, Y. Zhao, T.C. Sum, J. Lin, Z. Shen, Hierarchical porous LiNi1/3Co1/3Mn1/3O2 nano-/micro spherical cathode material: minimized cation mixing and improved Li+ mobility for enhanced electrochemical Performance. Sci. Rep. 6, 25771 (2016). https://doi.org/10.1038/srep25771
J. Hong, K. Kang, H. Gwon, S.K. Jung, K. Ku, Review—Lithium- excesslayered cathodes for lithium rechargeable batteries. J. Electrochem. Soc. 162, A2447–A2467 (2015). https://doi.org/10.1149/2.0071514jes
H. Pan, S. Zhang, J. Chen, M. Gao, Y. Liu, T. Zhu, Y. Jiang, Li- and Mn-rich layered oxide cathode materials for lithium-ion batteries: a review from fundamentals to research progress and applications. Mol. Syst. Des. Eng. 3, 748–803 (2018). https://doi.org/10.1039/C8ME00025E
N.A. Chernova, M. Ma, J. Xiao, M.S. Whittingham, J.C. Jiménez, C.P. Grey, Magnetic studies of layered cathode materials for lithium ion batteries. Mater. Res. Soc. Symp. Proc. 972, 610 (2007). https://doi.org/10.1557/PROC-0972-AA06-10
N.A. Chernova, M. Ma, M. Jie Xiao, Stanley Whittingham, Julien Breger, Clare P. Grey, Layered LixNiyMnyCo1–2yO2 cathodes for lithium ion batteries: understanding local structure via magnetic properties. Chem. Mater. 19, 482–4693 (2007). https://doi.org/10.1021/cm0708867
M.N. Ates, S. Mukerjee, K.M. Abraham, A high rate Li-rich layered MNC cathode material for lithium-ion batteries. RSC Adv. 5, 27375–27386 (2015). https://doi.org/10.1039/C4RA17235C
Y. Errami, M. Ouassaid, M. Cherkaoui, M. Maaroufi, Maximum power point tracking control based on a nonlinear back stepping approach for a permanent magnet synchronous generator wind energy conversion system connected to a utility grid. Ener. Technol. 3, 743–757 (2015). https://doi.org/10.1002/ente.201500026
D. Harbaoui, M.M.S. Sanad, C. Rossignol, E. Hlil, N. Amdouni, S. Obbade, Synthesis and structural, electrical, and magnetic properties of new iron–aluminum alluaudite phases β-Na2Ni2M(PO4)3 (M = Fe and Al). Inorg. Chem. 56, 13051–13061 (2017). https://doi.org/10.1021/acs.inorgchem.7b01880
N. Murali, K.V. Babu, K.E. Babu, V. Veeraiah, Structural and conductivity studies of LiNi0.5Mn0.5O2 cathode materials for lithium-ion batteries. Mater. Sci-Pol. 34, 404–411 (2016)
N. Murali, V. Veeraiah, Preparation, dielectric and conductivity studies of LiNi1-xMgxO2 cathode materials for lithium-ion batteries. Process. Appl. Ceram. 11, 258–264 (2017). https://doi.org/10.2298/PAC1704258M
N. Murali, S.J. Margarette, V. Veeraiah, Synthesis, dielectric, conductivity and magnetic studies of LiNi1/3Co1/3Mn(1/3)−xAlxO2 (x=0.0, 0.02, 0.04 and 0.06) for cathode materials of lithium-ion batteries. Results Phys. 7, 1379–1388 (2017). https://doi.org/10.1016/j.rinp.2017.02.037
H. Arai, S. Okada, Y. Sakurai, J.-I. Yamaki, Reversibility of LiNiO2 cathode. Solid State Ionics 95, 275–282 (1997). https://doi.org/10.1016/S0167-2738(96)00598-X
S.S. Zhang, K. Xu, T.R. Jow, Formation of solid electrolyte interface in lithium nickel mixed oxide electrodes during the first cycling. Electrochem. Solid-State Lett. 5, A92–A94 (2002). https://doi.org/10.1149/1.1464506
J.P. Peres, C. Delmas, A. Rougier, M. Broussely, F. Perton, P. Biensan, P. Willmann, The relationship between the composition of lithium nickel oxide and the loss of reversibility during the first cycle. J. Phys. Chem. Solids 57, 1057–1060 (1996). https://doi.org/10.1016/0022-3697(95)00395-9
C. Delmas, J.P. Peres, A. Rougier, A. Demourgues, F. Weill, A. Chadwick, M. Broussely, F. Perton, P. Biensan, P. Willmann, On the behavior of LixNiO2 system: an electrochemical and structural overview. J. Power Sources 68, 120–125 (1997). https://doi.org/10.1016/S0378-7753(97)02664-5
J.R. Mueller-Neuhaus, R.A. Dunlap, J.R. Dahn, Understanding irreversible capacity in Lix Ni1 − y Fey O 2 cathode materials. J. Electrochem. Soc. 147, 3598–3605 (2000)
J. Choi, A. Manthiram, Investigation of the irreversible capacity loss in the layered LiCo1/3Ni1/3Mn1/3O2 cathodes. Electrochem. Solid-State Lett. 8, C102–C105 (2005). https://doi.org/10.1149/1.1943567
A.Y. Shenouda, M.M.S. , Sanad, Synthesis, characterization and electrochemical performance of Li2NixFe1−xSiO4 cathode materials for lithium ion batteries. Bull. Mater. Sci. 40, 1055–1060 (2017). https://doi.org/10.1007/s12034-017-1449-2
A. Abdel-Aziz, A.Y. Shenouda, M.M.S. Sanad, B. Kishore, H.F.Y. Khalil, M.M.B. El-Sabbah, Effect of ionic substitutions on the physicochemical, morphological, and electrochemical properties of lithium-rich vanadium phosphate and pyrophosphate compounds. Ionics 25, 969–980 (2019). https://doi.org/10.1007/s11581-019-02843-7
Acknowledgements
The author is grateful and thankful to Science and Technology Development Fund (STDF) for the financial support of the work through German-Egyptian Research Fund (GERF) grant ID: 23038. Great thanks are extended to the The Hydrogen and Fuel Cell Center–Duisburg (Germany) for performing the rate capability and long-term cycling test of batteries under the assistance and supervision of Dipl.-Ing. Bernd Oberschachtsiek and Dr. George Topalov at the department of electrolysis and batteries. Deep acknowledgments are presented to Dr. Sebastian Wennig (Hydrogen and Fuel Cell Center-Duisburg-Germany) for providing language and scientific help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sanad, M.M.S., Toghan, A. Unveiling the role of trivalent cation incorporation in Li-rich Mn-based layered cathode materials for low-cost lithium-ion batteries. Appl. Phys. A 127, 733 (2021). https://doi.org/10.1007/s00339-021-04884-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04884-0