Abstract
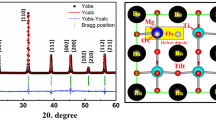
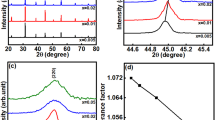
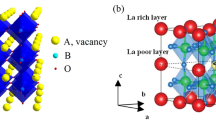
Garnet-structured solid electrolyte materials have drawn much attention due to their high ionic conductivity and electrochemical stability. Doping strategy is found to be very helpful in enhancing the performance of garnet-based solid electrolytes. In the present study, Li6.28Al0.24La3Zr2O12 with different amounts of Ba2+ doping at La3+ site is prepared with the help of solid-state reaction method. Li6.38Al0.24La2.9Ba0.1Zr2O12 sintered at 1000° C shows stabilization of cubic phase having closely packed grains with ionic conductivity of 0.46 × 10–4 S/cm at 25°C and activation energy of 0.29 eV. The presence of a well-defined thermally activated relaxation process in the prepared material is confirmed by the frequency-dependent electrical modulus and dielectric spectra.












Similar content being viewed by others
References
J. Sun, B. Mao, Q. Wang, Fire Saf. J. (2020). https://doi.org/10.1016/j.firesaf.2020.103119
Q. Wang, B. Mao, S.I. Stoliarov, J. Sun, Prog. Energy Combust. Sci. (2019). https://doi.org/10.1016/j.pecs.2019.03.002
J. Liang, J. Luo, Q. Sun, X. Yang, R. Li, X. Sun, Energy Storage Mater. (2019). https://doi.org/10.1016/j.ensm.2019.06.021
C. Wang, K.R. Adair, J. Liang, X. Li, Y. Sun, X. Li, J. Wang, Q. Sun, F. Zhao, X. Lin, R. Li, H. Huang, L. Zhang, R. Yang, S. Lu, X. Sun, Adv. Funct. Mater. (2019). https://doi.org/10.1002/adfm.201900392
Y. Chen, K. Wen, T. Chen, X. Zhang, Energy Storage Mater. (2020). https://doi.org/10.1016/j.ensm.2020.05.019
S. Chen, D. Xie, G. Liu, J.P. Mwizerwa, Q. Zhang, Y. Zhao, X. Xu, X. Yao, Energy Storage Mater. (2018). https://doi.org/10.1016/j.ensm.2018.02.020
D. Zhou, D. Shanmukaraj, A. Tkacheva, M. Armand, G. Wang, Chem (2019). https://doi.org/10.1016/j.chempr.2019.05.009
J. Huang, F. Liang, M. Hou, Y. Zhang, K. Chen, D. Xue, Appl. Mater. Today (2020). https://doi.org/10.1016/j.apmt.2020.100750
R. Murugan, V. Thangadurai, W. Weppner, Angew. Chem. Int. Ed. (2007). https://doi.org/10.1002/anie.200701144
C. Sun, Y. Ruan, W. Zha, W. Li, M. Cai, Z. Wen, Mater. Horiz. (2020). https://doi.org/10.1039/D0MH00050G
J. Awaka, N. Kijima, H. Hayakawa, J. Akimoto, J. Solid State Chem. (2009). https://doi.org/10.1016/j.jssc.2009.05.020
J. Awaka, A. Takashima, K. Kataoka, N. Kijima, Y. Idemoto, J. Akimoto, Chem. Lett. (2011). https://doi.org/10.1246/cl.2011.60
N. Bernstein, M.D. Johannes, PRL (2012). https://doi.org/10.1103/PhysRevLett.109.205702
K. Meier, T. Laino, A. Curioni, J. Phys. Chem. C (2014). https://doi.org/10.1021/jp5002463
C.A. Geiger, E. Alekseev, B. Lazic, M. Fisch, T. Armbruster, R. Langner, M. Fechtelkord, N. Kim, T. Pettke, W. Weppner, Inorg. Chem. (2011). https://doi.org/10.1021/ic101914e
C.L. Li, Y. Liu, J. He, K.S. Brinkman, J. Alloy. Compd. (2017). https://doi.org/10.1016/j.jallcom.2016.11.277
L. Dhivya, K. Karthik, S. Ramakumar, R. Murugan, RSC Adv. (2015). https://doi.org/10.1039/C5RA18543B
E. Rangasamy, J. Wolfenstine, J. Sakamoto, Solid State Ion. (2012). https://doi.org/10.1016/j.ssi.2011.10.022
J.F. Wu, E.Y. Chen, Y. Yu, L. Liu, Y. Wu, W.K. Pang, V.K. Peterson, X. Guo, A.C.S. Appl, Mater. Interfaces (2017). https://doi.org/10.1021/acsami.6b13902
L. Dhivya, N. Janani, B. Palanivel, R. Murugan, AIP Adv. (2013). https://doi.org/10.1063/1.4818971
S. Ohta, T. Kobayashi, T. Asaoka, J. Power Sources (2011). https://doi.org/10.1016/j.jpowsour.2010.11.089
S. Song, B. Chen, Y. Ruan, J. Sun, L. Yu, Y. Wang, J. Thokchom, Electrochim. Acta (2018). https://doi.org/10.1016/j.electacta.2018.03.101
R. Inada, K. Kusakabe, T. Tanaka, S. Kudo, Y. Sakurai, Solid State Ionics (2014). https://doi.org/10.1016/j.ssi.2013.09.008
C. Shao, Z. Yu, H. Liu, Z. Zheng, N. Sun, C. Diao, Electrochim. Acta (2017). https://doi.org/10.1016/j.electacta.2016.12.140
E. Rangasamy, J. Wolfenstine, J. Allen, J. Sakamoto, J. Power Sources (2013). https://doi.org/10.1016/j.jpowsour.2012.12.076
C. Deviannapoorani, L.S. Shankar, S. Ramakumar, R. Murugan, Ionics (2016). https://doi.org/10.1007/s11581-016-1674-5
A. Dumon, M. Huang, Y. Shen, C.W. Nan, Solid State Ion. (2013). https://doi.org/10.1016/j.ssi.2013.04.016
D. Wang, G. Zhong, O. Dolotko, Y. Li, M.J. McDonald, J. Mi, R. Fud, Y. Yang, J. Mater. Chem. (2014). https://doi.org/10.1039/c4ta03591g
D.O. Shin, K. Oh, K.M. Kim, K.Y. Park, B. Lee, Y.G. Lee, K. Kang, Sci. Rep. (2015). https://doi.org/10.1038/srep18053
T. Yang, Y. Li, W. Wu, Z. Cao, W. He, Y. Gao, J. Liu, G. Li, Ceram. Int. (2018). https://doi.org/10.1016/j.ceramint.2017.10.072
Y. Meesala, Y.K. Liao, A. Jena, N.H. Yang, W.K. Pang, S.F. Hu, H. Chang, C.E. Liu, S.C. Liao, J.M. Chen, X. Guo, R.S. Liu, J. Mater. Chem. A (2019). https://doi.org/10.1039/C9TA00417C
Y. Luo, X. Li, Y. Zhang, L. Ge, H. Chen, L. Guo, Electrochim. Acta (2019). https://doi.org/10.1016/j.electacta.2018.10.078
J.F. Wu, W.K. Pang, V.K. Peterson, L. Wei, X. Guo, A.C.S. Appl, Mater. Interfaces (2017). https://doi.org/10.1021/acsami.7b00614
X. Li, R. Li, S. Chu, K. Liao, R. Cai, W. Zhou, Z. Shao, J. Alloy. Compd. (2019). https://doi.org/10.1016/j.jallcom.2019.04.274
J. Gai, E. Zhao, F. Ma, D. Sun, X. Ma, Y. Jin, Q. Wu, Y. Cui, J. Eur. Ceram. Soc. (2018). https://doi.org/10.1016/j.jeurceramsoc.2017.12.002
B.P. Dubey, A. Sahoo, V. Thangadurai, Y. Sharma, Solid State Ion. (2020). https://doi.org/10.1016/j.ssi.2020.115339
W. Gu, M. Ezbiri, R.P. Rao, M. Avdeev, S. Adams, Solid State Ion. (2015). https://doi.org/10.1016/j.ssi.2015.03.019
Y. Jin, P.J. McGinn, J. Power Sources (2011). https://doi.org/10.1016/j.jpowsour.2011.05.065
R.D. Shannon, Handbook of Chemistry and Physics, 74th edn. (CRC Press, Boca Raton, FL, 1974)
A.K. Jonscher, Dielectric Relaxation in Solids (Chelsea Dielectric Press, London, 1983)
A. Sodhiya, R. Kumar, S. Patel, A.K. Singh, S. Soni, AIP Conf. Proc. (2020). https://doi.org/10.1063/5.0019544
S. Ramakumar, L. Satyanarayana, S.V. Manorama, R. Murugan, Phys. Chem. Chem. Phys. (2013). https://doi.org/10.1039/C3CP50991E
J.H. Joshia, D.K. Kanchan, M.J. Joshi, H.O. Jethva, K.D. Parikh, Mater. Res. Bull. (2017). https://doi.org/10.1016/j.materresbull.2017.04.013
D.M. Abdel-Basset, S. Mulmi, M.S. El-Bana, S.S. Fouad, V. Thangadurai, Inorg. Chem. (2017). https://doi.org/10.1021/acs.inorgchem
J.R. Macdonald, Impedance Spectroscopy (Wiley, New York, USA, 1987)
Acknowledgements
Authors wish to acknowledge sophisticated instrument center (SIC) for XRD, SEM characterization and department of chemistry for impedance measurement of Dr. Harisingh Gour Vishwavidyalaya, Sagar (MP), India.
Funding
This work was supported by UGC non-NET fellowship.
Author information
Authors and Affiliations
Contributions
RK has made substantial contribution in conceptualization and design of experiment, and AKS and SS have substantial contribution in acquisition and interpretation of data. AS has contribution in conceptualization, acquisition and interpretation of data and drafting the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
sodhiya, A., kumar, R., singh, A.k. et al. Effect of Ba2+ doping on the structure and transport properties of Li6.28Al0.24La3Zr2O12 solid electrolyte. Appl. Phys. A 127, 584 (2021). https://doi.org/10.1007/s00339-021-04729-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04729-w