Abstract
Human-induced climate change is causing ocean warming that triggers the breakdown of the coral–algal symbiosis. The proximate cause of this phenomenon, known as coral bleaching, is commonly attributed to the overproduction of reactive oxygen species (ROS) by the thermally stressed photosynthetic algal symbionts. However, direct evidence that algal ROS production (e.g., in the form of H2O2) and coral physiological stress are the ultimate cause of bleaching remains ambiguous. Here, we investigated the temporal dynamics of H2O2 and oxygen (O2) concentrations during thermally induced coral bleaching to disentangle cause from consequence. Microsensors at the tissue interface of Pocillopora damicornis measured H2O2 and O2 concentrations while exposing single nubbins to baseline temperatures (30 °C) and to minor (33 °C), moderate (36 °C), and high (39 °C) levels of acute heat stress using the Coral Bleaching Automated Stress System (CBASS). We show that a temporary decline in O2 concentration, accompanied by a declining photosynthetic efficiency and loss of Symbiodiniaceae and pigmentation, is the initial response to moderate thermal stress. This response was neither provoked nor followed by an increased H2O2 concentration at the coral tissue interface. A steady light-independent increase of H2O2 was only detected during high heat stress, resulting in the complete and permanent loss of photosynthetic activity. Our findings do not support a direct connection between algal photodamage and an increase in H2O2 concentration during thermally induced bleaching and suggest that more research on the function of H2O2 is warranted. This notion is further substantiated by the observation of an additional source of H2O2, likely oxidative bursts, that were common at the baseline temperature and under minor heat stress, while their occurrence decreased at moderate and high heat stress. Resolving the multifaceted and dynamic roles of H2O2 in coral bleaching is critical to better understand the response of the coral holobiont to thermal stress and identifying the processes underlying the breakdown of the coral–algal symbiosis.
Similar content being viewed by others
Introduction
Global warming is affecting biota with consequences for virtually every ecosystem on Earth. Tropical coral reefs are particularly threatened by ocean warming (Eakin et al. 2022), ocean acidification (Cornwall et al. 2021), deoxygenation (Hughes et al. 2020), and salinity changes (Röthig et al. 2023; Coles and Jokiel 1992). Reef-building corals and their symbioses with photosynthetic dinoflagellates and other microbes (i.e., the coral holobiont) are the foundation of tropical reef ecosystems that have significant ecological and economic value (Costanza 2014). Ocean warming is currently the most pressing threat, as it causes the (repeated) expulsion of the endosymbiotic dinoflagellates leading to coral bleaching and often to subsequent coral mortality (Hughes et al. 2017).
Although coral bleaching is extensively studied (e.g., Glynn 1993; Brown 1997; Warner et al. 1999), its underlying mechanism and what “pulls the trigger” are still the subject of ongoing debate (Rädecker et al. 2023; Dungan et al. 2022; Rädecker et al. 2021; Suggett and Smith 2020). Nevertheless, a common notion is that the production and accumulation of excessive photosynthetically derived reactive oxygen species (ROS) due to heat stress lead to cellular damage and bleaching (Lesser 1997, 2011; Downs et al. 2002).
ROS such as superoxide anion radicals (O2−), hydrogen peroxide (H2O2), hydroxyl radical ions (.OH), or hydroxyl radical ions (OH−) arise as a consequence of single electron transfer reactions with oxygen (Byczkowski and Gessner 1988). Despite extensive investigations of ROS production during bleaching, the particular ROS involved remains ambiguous. However, H2O2 has been suggested as the most important ROS associated with coral bleaching (Downs et al. 2002; Smith et al. 2005; Solayan 2016). H2O2 functions as an important signaling molecule between the coral host and its algal symbiont, due to its long lifetime and ability to rapidly diffuse through cells and across membranes, which make it a key molecule within the coral holobiont (Roberty et al. 2015; Smith et al. 2005). Moreover, the prevalent ROS O2− is highly reactive and rapidly converted into H2O2 by the antioxidant enzyme superoxide dismutase. Thus, external levels of H2O2 at the coral tissue interface are also the result of internal and external O2− production and conversion rates (Hansel and Diaz 2021; Ousley et al. 2022).
Within the coral holobiont, O2− and H2O2 can be produced by the coral host, the photosynthetic algal symbiont, and associated bacteria, whereas at the cell biological scale, the locations and pathways are diverse (Hansel and Diaz 2021). H2O2 plays an essential role in cellular homeostasis, health, and signaling and is released in bursts that are characterized by rapid increases and decreases lasting seconds to minutes in non-stressed corals (Ousley et al. 2022). Oxidative bursting is caused by specific enzymes such as the NADPH oxidase (NOX), belonging to the family of transmembrane oxidoreductases (Suzuki et al. 2011; Hansel and Diaz 2021). Notably, H2O2 is an inevitable byproduct of aerobic metabolic processes such as respiration and photosynthesis. However, H2O2 concentrations above organism-specific thresholds can cause oxidative stress and be harmful to cells (Fridovich 1998; Aguirre et al. 2005; Lesser 2006; Hopkins 2016). Given the multifunctional roles of ROS, intracellular concentrations are tightly regulated through antioxidant molecules and enzymes such as superoxide dismutase, catalase, and ascorbate peroxidase (Fridovich 1998). Catalase is an important heme-based metalloenzyme that scavenges H2O2 by splitting it into water and O2, preventing the formation of hydroxyl radicals, a highly reactive form of reduced oxygen (Apel and Hirt 2004).
Under thermal stress, the equilibrium between ROS production and degradation in the coral holobiont can be disturbed resulting in increased ROS levels (Weis 2008; Lesser 2011). Occasional single electron redox reactions in the mitochondrial electron transport chain and the photosynthetic system will lead to the formation of singlet oxygens (Apel and Hirt 2004). More specifically, heat stress can damage the photosystem II and the associated D1 protein, which can result in photoinhibition and the uncoupling of the electron flow, when exceeding the natural repair capacity of the organism (Weis 2008). It has also been suggested that heat stress can directly damage the thylakoid membrane and the ribulose bisphosphate carboxylase oxygenase (RuBisCO) enzyme, which results in decreased ATP and NADPH consumption and ultimately enhances the buildup of electrons (Weis 2008). Moreover, single electron transfers at the photosystem I can result in the formation of O2− that can be further reduced to H2O2, known as the Mehler reaction (Tchernov et al. 2004).
The oxidative theory of coral bleaching (Downs et al. 2002) posits that increasing temperatures cause damage to the photosystem II of the algal symbiont, resulting in photoinhibition and the overproduction of H2O2 that leaks into the coral host tissue (Lesser 1997; Tchernov et al. 2011; Weis 2008). With increasing oxidative stress, the ROS scavenging activity in the coral host increases (Gardner et al. 2017; Krueger et al. 2015). When the thermal threshold of the coral host is exceeded, the defense mechanisms are overwhelmed by ROS leading to DNA, protein, and membrane damage (Richier et al. 2005; Lesser and Farrell 2004), eventually resulting in apoptosis and tissue necrosis (Gates et al. 1992). It has been hypothesized that the last attempt of the coral to reduce further oxidative stress by the algal symbiont causes the breakdown of the mutualistic relationship and expulsion of the algal symbiont, i.e., bleaching (Dunn et al. 2004; Tchernov et al. 2011; Hawkins et al. 2013).
Recent findings, however, challenge ROS as the primary driver of coral bleaching (Dungan et al. 2022; Nielsen et al. 2018) and suggest the breakdown of metabolic cycling precedes oxidative damage and may be the ultimate pre-requisite of bleaching (Rädecker et al. 2023; Rädecker et al. 2021). Nevertheless, direct evidence for this is scarce and heat stress responses may be particularly complex in holobionts (Rädecker et al. 2021). Each holobiont member may differ in their response to heat stress, responses may also vary across coral species and even among polyps within a coral colony (Voolstra et al. 2021). Therefore, further clarifying the sequence of events in which different tissue-scale processes occur and eventually lead to coral bleaching can substantially advance knowledge on thermal stress biology across biological scales (Van Woesik et al. 2022).
Here we aim to better understand the role of H2O2 in coral bleaching by determining the dynamics of H2O2 concentration during thermal stress at high temporal resolution directly at the coral tissue interface. This was achieved by exposing Pocillopora damicornis nubbins to a series of acute thermal stress assays using the Coral Bleaching Automated Stress System (CBASS) (Evensen et al. 2023; Voolstra et al. 2021, 2020), while monitoring H2O2 and O2 dynamics on single polyps using microsensors. Specifically, we investigated (1) how the occurrence of oxidative bursts in the holobiont changes with increasing thermal stress, (2) whether inhibition of photosynthesis is the initial response to thermal stress that triggers increased H2O2 concentrations or vice versa, and (3) if photosynthetically produced H2O2 ultimately leads to the cessation of photosynthetic activity under high heat stress (39 °C).
Materials and methods
Coral rearing
Nubbins of a P. damicornis colony, originally sourced from and reared at the Royal Burgers’ Zoo in Arnhem (Netherlands), were transferred to the MAREE facilities of the Leibniz Centre for Tropical Marine Research (ZMT) in 2018. Coral nubbins were maintained in artificial seawater (ASW) prepared from Red Sea salt and reverse osmosis water and reared in a 180 L aquarium. The coral nubbins were held at a constant water temperature of 25.4 °C (SD = 0.07 °C), a salinity of 35.1 PSU (SD = 0.03 PSU), and at a light intensity of 138 µmol photons m−2 s−1 (LI-250A Light Meter, WALZ) supplied by 3 blue LED panels and 3 white LED panels (Daytime, Waltrin GmbH) in a 12:12-h dark/light cycle. A total of 16 P. damicornis nubbins (0.5–2 cm in length) were obtained from the same colony (4 replicates × 4 acute thermal stress profiles). Nubbins were glued onto holders and allowed to recover for at least one week prior to the CBASS assays.
Measurement of H2O2 concentration dynamics during acute thermal stress assays
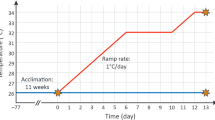
Coral nubbins were transferred from the rearing aquarium to the 10L CBASS holding tanks. All CBASS assays were conducted in a climate-controlled room at the Biology Laboratory of the ZMT. Real-time tracking microsensors measured H2O2 and O2 concentrations at the coral tissue interface of single polyps from single nubbins, while running acute thermal stress profiles using the Coral Bleaching Automated Stress System (CBASS) (four replicates for each of the four thermal stress profiles). Water temperature was recorded every second using a HOBO logger (Pendant, MX2201). A CBASS assay comprises four independent temperature profiles with one 19h long 30°C baseline hold profile and three distinct heat stress cycling profiles of a 3h ramp up, 3h heat-hold (33 °C minor heat stress, 36 °C moderate heat stress and 39 °C high heat stress), 1h ramp down, and a 12h overnight hold at baseline temperature (Fig. 1). The baseline temperature of 30 °C was chosen based on pilot CBASS assays at 27 °C and 30 °C (Supplementary Fig. S1). Coral nubbins were placed into CBASS tanks and temperature was increased from the rearing temperature to 30 °C over a 30-min interval before the start of the 19h long CBASS temperature profiles. At the beginning of the ramp-down phase, the white light was switched off, and after a further 30 min the blue light was switched off (night cycle). Photosynthetic efficiency was measured after 30 min of dark acclimation (Fig. 1). The light remained switched off during nighttime until the next day at 07:00 am. Upon completion of the CBASS run at 08:00 am, nubbins were removed from the tanks, photographed for visual bleaching assessment using a Canon G15 camera (Canon Zoom Lens 5 × IS) against a color reference card, and transferred into 1 M NaOH for Symbiodiniaceae cell counts. To assess the effect of light on H2O2 concentration dynamics, we also conducted the 39 °C heat stress cycling profile during the night (ramp up from 01:00 am to 04:00 am, heat-hold phase from 04:00 am to 07:00 am)
Temperature profiles maintained using the Coral Bleaching Automated Stress System (CBASS) to run a standardized short-term acute heat stress assay (Voolstra et al. 2020). A CBASS assay consists of a baseline temperature profile at 30 °C (blue line) and three thermal stress profiles consisting of 3h thermal ramps to 3h heat-hold phases at 33 °C (orange line), 36 °C (red line), and 39 °C (dark red line), a 1h ramp-down phase, and a subsequent 12-h holding phase at the baseline temperature. After the end of the ramp-down phase (1), photosynthetic efficiency was measured, and at the end of the CBASS cycle (2) all coral nubbins were photographed for a visual inspection of bleaching extent.
Photosynthetic efficiency
Photosynthetic efficiency was measured per nubbin as the dark-acclimated maximum quantum yield (Fv/Fm) of photosystem II using a diving pulse amplitude modulated (PAM) fluorometer (Walz), following the ramp-down phase (Fig. 1, Table S4). Fluorescence level and gain settings were adjusted before measurements (Meas. Int. 6; Gain 2; Damp 3; Sat Int. 8 Ft ~ 300 to 500).
Visual bleaching assessment and Symbiodiniaceae cell count
Bleaching extent was assessed based on photographs of coral nubbins in comparison to a color reference card. The degree of bleaching was classified into six pigmentation categories: 100%, 80%, 60%, 40%, 20%, and 0%. Three people scored the extent of bleaching across each nubbin independently and the mean was calculated (Table S5) (Morikawa and Palumbi 2019; Alderdice et al. 2022).
For Symbiodiniaceae cell counts, coral nubbins from CBASS assays were used. Coral tissue was removed from the underlying skeleton using the sodium hydroxide (NaOH) method (Zamoum and Furla 2012; Pogoreutz et al. 2017). Each coral nubbin was incubated for 1 h in 1 M NaOH to dissolve the tissue. The skeleton was then removed to measure the coral surface area of each nubbin. The suspended Symbiodiniaceae cells were washed three times with 1 mL 1 × Phosphate-Buffered Saline and spun down in a bench-top centrifuge (5 min, 3000 RCF, 25 °C). A Neubauer counting chamber was used to determine the number of cells within the washed cell pellet from each coral nubbin. The surface area of the skeleton was determined using the geometric approximation method (Naumann et al. 2009). Each nubbin was categorized into a specific shape (e.g., cylinder, cuboid), a caliper was used to measure dimensions, and the respective geometric formulas were used to normalize Symbiodiniaceae cell counts to coral surface area (cm2) (Table S6).
Hydrogen peroxide and oxygen microsensor measurement
H2O2 and O2 microsensors were built by the Microsensor group at the Max-Planck Institute for Marine Microbiology. The O2 microsensors with an internal reference were constructed based on the Clark-type oxygen microelectrodes (Revsbech and Jørgensen 1986). H2O2 microsensors were constructed as an adaptation of a previous glucose microsensor (Cronenberg et al. 1991). Because of the significant cross-sensitivity of the microsensor to nitric oxide (NO) (49-63%) (Ousley et al. 2022), NO had to be considered part of the measured H2O2 signal. Signal interferences were removed from measured microsensor signals by calculating the second derivative of concentration over time. Interferences were defined as cases where the absolute value was > 5 µM s−1. This threshold was chosen after visual inspection of a number of bursts and interferences. All data points logged shortly after each interference were removed until the signal returned (+ 5 µM s−1) to the baseline value (defined as the median of the 10 data points before the interference). The removal of interference signals was conducted in R and was run twice to ensure discrimination between interferences and bursts (Figs. S2–S5). Both microsensors were calibrated before each CBASS run as described in Ousley et al. (2022). For the calibration of the H2O2 microsensor, the signal of the ASW was used for the 0 µM concentration. A prior experiment revealed a potential offset of 0.3 µM H2O2 between the ASW baseline and the true zero H2O2 baseline (ASW + catalase). However, the signal of the ASW was used as the 0 µM baseline, since a true zero baseline would require the addition of catalase to the calibration beaker as well as the CBASS tank, which could have impacted the coral as well as the H2O2 production. In addition, one may argue that the offset is negligible compared to the H2O2 concentrations reached during bursting. To improve microsensor accuracy, an offset correction of the calibration was applied if the readings differed between the water column (0 µM) of the calibration beaker and the water column (0 µM) of the CBASS tank. The difference in the microsensor signal was added to each calibration step since we assumed an overall shift in the signal.
CBASS microsensor setup
The original CBASS setup was adjusted to allow for the assembly and positioning of the microsensors at the coral tissue interface of single nubbins (Fig. 2). A 2L flow-through chamber was placed into the 10L CBASS holding tank to reduce electrical interferences provoked by the chillers and heater (Fig. 2). The O2 and H2O2 microsensors as well as the H2O2 references and grounding wire were placed in the 2L chamber (Fig. 2). Each microsensor was held by a micromanipulator and connected through a custom-made picoamperemeter and a DAQ-Pad to a computer for data transmission (Fig. 2). The heater, the chillers, a flow pump, and the sensor of the temperature controller were placed in the 10L holding tank, as in the original CBASS setup (Voolstra et al. 2020). The accuracy of all temperature profiles was assayed using a HOBO logger placed inside the 2L chamber. The chamber was illuminated from above by one LED light with 50% blue light and 50% white light matching the long-term rearing light intensity of ~ 138 µmol photons m−2 s−1 (LI-250A Light Meter, WALZ) (Fig. 2).
CBASS microsensor setup to measure H2O2 and O2 at the coral tissue interface. A 2L flow-through chamber (2) with an input (25 ml min−1) and output for ASW was placed into one 10L CBASS holding tank (1). The H2O2 (3) and the O2 (4) microsensors were held by micromanipulators (5) on each side of the tank. An external reference for the H2O2 microsensor and a grounding wire were placed inside the chamber (6). Each microsensor was connected to the computer through a custom-made picoamperemeter (7) and a DAQ-Pad (8) for data transmission. Above the chamber, one LED light (9) provided illumination. The heater, the chillers, a flow pump, and the sensor of the temperature controller were placed inside the 10-L holding tank (not shown). A HOBO logger was placed inside the 2L flow-through chamber for temperature data collection (not shown). The zoomed-in picture shows a coral nubbin and the two microsensors positioned at the coral tissue interface
Statistical analysis
All data were assessed for normal distribution and homoscedasticity prior to ANOVA testing with subsequent Tukey’s post hoc tests. To identify the baseline temperature to use during the CBASS assay, ANOVAs were conducted to assess whether photosynthetic efficiency was significantly different between the rearing temperature (25 °C) and the 27 °C or 30 °C baseline temperature used in the pilot CBASS assays (Table S1). ANOVAs were also conducted to test for significant differences in photosynthetic efficiency, coral pigmentation, and Symbiodiniaceae cell numbers among the four CBASS thermal profiles (Table S2). To determine standardized thermal thresholds, i.e., ED50s (Evensen et al. 2023; Evensen et al. 2021; Voolstra et al. 2021) based on photosynthetic efficiency, the effective dose model of the DRC package in R was used (Ritz and Streibig 2005) (Fig. S6). The ED50 value defines the temperature at which the coral still retains 50% of the photosynthetic efficiency it had at the baseline temperature.
Results
Heat stress level affects the occurrence of oxidative bursts and baseline H2O2 concentration
At the baseline temperature (30 °C) and under minor (33 °C) and moderate heat stress (36 °C), dynamic oxidative bursting of H2O2 occurred (Fig. 3a). Oxidative bursting was highly variable during the day and during the night and among coral replicates (Figs. S7–S9). At moderate heat stress, we observed a trend toward less oxidative bursting compared to the baseline temperature and minor heat stress (Figs. 3a, S9). This decrease became even more apparent under high heat stress, with even less frequent bursting across replicates (Figs. 3a, S10). The baseline concentration of H2O2 increased irregularly between 2 and 7 µM at 30 °C and under minor heat stress of 33 °C (Figs. 3a, S7-S8). In comparison, at moderate heat stress of 36 °C, we detected a trend toward a lower baseline concentration of H2O2 (Figs. 3a, S9, S11). The baseline H2O2 concentration mostly increased during daytime across all three temperatures (30 °C, 33 °C, 36 °C) (Figs. 3a, S7–S9). Under high heat stress of 39 °C, a steady and steep increase in the baseline H2O2 concentration was detected after 3 h of heat exposure (Fig. 3a, S10). H2O2 concentration steadily increased for approximately 3 h until reaching a maximum concentration between 7 µM and 54 µM before decreasing again over the course of 2 h (Fig. S10).
Overview of microsensor readings over time and physiological metrics of the coral nubbins across the four distinct acute thermal stress temperature profiles. a Light (yellow) and temperature (gray) curves as well as H2O2 (red) and O2 (blue) concentrations for the respective CBASS runs (30 °C, 33 °C, 36 °C, 39 °C) are shown; measurement of a single coral coral nubbin (n = 4 in total) is shown. Dashed lines indicate the time points when the white light (at 19.00h) and the blue light (at 19.30h) were switched off and when all lights were switched back on the next morning (at 07.00h). O2 concentration decreased through the night and subsequently increased during daytime at 30 °C and 33 °C. At 36 °C, O2 concentration decreased before nighttime but partially recovered throughout the night (see, partial increase of O2 concentration the next morning). At 39 °C, O2 concentration also decreased before nighttime but without recovery the following morning. b Violin and boxplots show the values of photosynthetic efficiency, coral pigmentation, and Symbiodiniaceae cell numbers observed across the four thermal stress temperature profiles
Exposure to moderate and high heat stress induces coral bleaching
When exposed to the baseline temperature (30 °C) or minor heat stress (33 °C), P. damicornis produced high concentrations of O2 during daytime conditions, highlighting that the endosymbionts remained photosynthetically active (Fig. 3a). Although the O2 concentration fluctuated during daytime, it decreased with decreasing light intensity (Fig. 3a, 19.00h) and increased with increasing light intensity the next day (Fig. 3a, 07.00h). Additionally, no significant decrease in photosynthetic efficiency, coral pigmentation, or Symbiodiniaceae cell number was detected (Fig. 3b, Table S2). During the thermal ramp up to moderate (36 °C) and high (39 °C) heat stress, O2 concentrations were initially high (fluctuating between 300 and 500 µM) but started to decline when the temperature reached approximately 35 °C during daytime (Figs. 3a, S9–S10). While after moderate heat stress the O2 concentration partially recovered as the light was switched on the next day, no recovery was detected after high heat stress. Moderate heat stress resulted in the significant reduction of photosynthetic efficiency by 48.3% (p = < 0.001), a decrease in coral pigmentation by 37.8% (p = 0.083), and a loss of Symbiodiniaceae cell numbers by 37.9% (p = 0.383) compared to the baseline temperature, all of which are indicative of coral bleaching (Fig. 3b, Tables S2-S3). High heat stress resulted in the significant reduction of photosynthetic efficiency by 88.7% (p = < 0.001), a significant decrease in coral pigmentation by 54.1% (p = 0.011), and in a loss of Symbiodiniaceae cell numbers by 53.1% (p = 0.145) compared to the baseline temperature (Fig. 3b, Tables S2–S3). Furthermore, tissue sloughing was detected after high heat stress.
Light-independent increase of H2O2 concentration during high heat stress
The steady H2O2 increase observed during exposure to high heat stress (39 °C) occurred after the O2 concentration declined when temperatures were above ~ 36 °C (Fig. 4a). Intriguingly, the same pattern of steady increase in H2O2 concentration was observed under high heat stress and at night (Fig. 4b). In the presence of light as well as in the dark, H2O2 concentration started to increase 3 h after the heat exposure and peaked between 45 and 50 µM (Fig. 4a, b). The steady increase in H2O2 concentration was detected before the light was switched on the next morning (Fig. 4b, first dashed line). Switching the light on at 07.00h did not impact the steady increase of H2O2 concentration (Figs. S13–S18). Hence, this excessive and continuous net H2O2 release is light-independent and thus decoupled from photosynthesis and even from photosynthetically derived O2. H2O2 concentration reached their maxima ~ 6 h into the thermal stress profile (Fig. S10) and consistently decreased afterward. Switching the light off (Fig. 4b, second dashed line) did not impact the already decreasing H2O2 concentration (Figs. S13, S15–S19). Furthermore, holding the temperature at 39 °C for an additional hour and increasing it again after returning to the baseline value (30 °C) did not result in a further net H2O2 increase (Fig. 4b).
Comparison of H2O2 and O2 concentration dynamics under high heat stress (39 °C) during day- and nighttime. Microsensor measurements of a representative coral nubbin from a 39 °C CBASS run a during the day (n = 4) and b during the night (n = 1). Light (yellow) and temperature (gray) curves as well as H2O2 (red) and O2 (blue) concentrations are shown. Dashed lines indicate the time points when the white light (at 19.00h) and the blue light (at 19.30h) were switched off and when all lights were switched back on the next morning (at 07.00h) and additionally switched back off after the night run (at ~ 08.18h , 4b). a O2 concentration decreased during daytime without recovery the following morning. H2O2 concentration started to increase at 16.00h after ~ 3 h of thermal stress (heat-hold) and peaked around 19.00h at ~ 45 µM before decreasing again. b O2 concentration decreased with decreasing light availability without a subsequent increase the next day. H2O2 concentration started to increase at 04.00h after ~ 3 h of thermal stress (heat-hold) and peaked at 07.00h at ~ 50 µM before decreasing again. Switching the light on (07.00h) and holding the heat stress temperature for an additional hour- as well as increasing it again to 39 °C had no further impact on H2O2 concentration
Discussion
Excessive photosynthetically produced ROS has long been flagged as the major cause of coral bleaching (Downs et al. 2002; Lesser 2011; Tchernov et al. 2011). Specifically, elevated concentrations of H2O2 detected during heat stress and the overproduction of H2O2 have been suggested as the main cause for the expulsion of the Symbiodiniaceae (Lesser 1996; Downs et al. 2002; Roberty et al. 2015). However, more recently, several studies have suggested ROS as the proximate ("smoking gun") rather than the ultimate cause of coral bleaching, since unequivocal evidence confirming a direct relationship is missing (Nielsen et al. 2018; Rädecker et al. 2021; Suggett and Smith 2020). Here, we present the first microsensor-based approach to disentangle the chronological sequence of the changes in O2 and H2O2 concentration during thermally induced bleaching. While bleaching is detected during moderate heat stress, elevated H2O2 concentrations were only observed under high heat stress, following a decline in O2 concentration leading to tissue sloughing and coral mortality. Although our study was conducted on aquarium-reared corals with limited biological replication, we argue that recording fine-scale data using microsensors at unprecedented temporal resolution during standardized heat stress assays provides novel insight into the timing and sequence of events underlying coral bleaching. Our study questions the direct connection between photodamage and H2O2 production at large and thus challenges the notion of ROS even being the “smoking gun” of bleaching.
H2O2 dynamics during heat stress
Across temperature treatments, we observed two types of H2O2 dynamics: (i) high frequent and dynamic oxidative bursting at the coral tissue interface when exposed to the baseline temperature (30 °C) as well as to minor (33 °C) and moderate (36 °C) heat stress (Fig. 3a), and (ii) a steady increase in H2O2 concentration during high (39 °C) heat stress (Fig. 3a). Our data support that oxidative bursting is common in healthy corals and potentially a distinctive feature of functioning coral holobionts (Ousley et al. 2022). Furthermore, we show that coral bleaching by means of coral pigmentation loss and Symbiodiniaceae cell loss (Fig. 3b) is already apparent under moderate heat stress (Voolstra et al. 2020) when oxidative bursting decreases compared to the baseline temperature and minor heat stress (Fig. 3a). In contrast, no oxidative bursting but a steady light-independent increase in H2O2 concentration occurred during high heat stress after the O2 concentration had declined (Figs. 3a, 4). The lack of O2 concentration recovery, the further decline in photosynthetic efficiency, together with the recorded loss of coral pigmentation, reduced number of Symbiodiniaceae cells, and tissue sloughing indicate the loss of photosynthetic activity and coral mortality (Fig. 3a, b).
We expected high background concentrations of H2O2 suggesting H2O2 production through photosynthetic mechanisms such as the Mehler reaction (Falkowski et al. 1985). Instead, we detected highly frequent oxidative bursting only at 30 °C, 33 °C, and 36 °C independently from the presence or absence of light as reported by Ousley et al. (2022) (Fig. 3a). The effect of light (decoupled from the effect of temperature) is clear when examining the dynamics of H2O2 concentration during the baseline temperature profile which was kept at 30 °C throughout the CBASS assay and where frequent oxidative bursting was detected during both day and night (Fig. 3a, S12). These findings highlight that other light-independent mechanisms contribute to the production of H2O2 within the coral holobiont. A plausible source of the observed bursts is the membrane-bound NADPH oxidase, which catalyzes the production of superoxide (O2−) and can be found in the coral host, algal symbionts, and bacteria (Hansel and Diaz 2021; Saragosti et al. 2010). Since oxidative bursting was high at 30 °C and 33 °C but decreased with increasing thermal stress (36 °C and 39 °C), we assume bursting is a sign of a healthy coral holobiont (Ousley et al. 2022). This is supported by concomitant active photosynthesis and stable photosynthetic efficiency, coral pigmentation, and Symbiodiniaceae cell numbers of coral nubbins exposed to the baseline temperature and to minor heat stress (Fig. 3a, b). O2− and H2O2 occur at the surface of healthy coral colonies (Saragosti et al. 2010; Ousley et al. 2022) and genes associated with ROS are involved in coral thermotolerance and resistance against pathogens (Dixon et al. 2015; Libro et al. 2013). Considering the role of H2O2 in cellular homeostasis, health, and signaling, the coral holobiont likely evolved diverse mechanisms to produce and scavenge H2O2 (Fridovich 1998; Hansel and Diaz 2021). Moreover, the consistent occurrence of oxidative bursts at the baseline temperature as well as under minor and moderate heat stress indicates that H2O2 was not, or at least not entirely, scavenged by the coral holobiont. However, we cannot make a definite claim about the gross rate of H2O2 production and the scavenging activity within the coral holobiont. Nevertheless, the presence of H2O2 at the baseline temperature and under minor heat stress as well as the absence of stress signs implies that oxidative bursting is not harmful to the organism. These findings show that the mere presence of H2O2 is not necessarily a hallmark for stress and must be interpreted with caution. Hence, oxidative bursting in coral holobiont physiology during non-stressful conditions warrants further investigation to eventually disentangle the diverse roles of H2O2 in holobiont health and functioning.
The occurrence of oxidative bursting and maximum concentrations of H2O2 were highly variable across replicates (i.e., single polyps of a nubbin replicate), corroborating earlier findings (Ousley et al. 2022). Such variation likely comes from spatial differences in the abundance and momentary strength of sinks and sources of H2O2 across the coral surface (Hansel and Diaz 2021). While polyp-scale data are lacking to confirm within coral colony variability, differences in the distribution of Symbiodiniaceae, bacteria, and mucus-associated microbes (among others) of the coral holobiont that possess NADPH oxidase activity have been found, which may contribute to the variability across polyps (Rohwer et al. 2002; Daniels et al. 2011; Hansel and Diaz 2021). Furthermore, NADPH availability may impact the production of O2− and therefore the occurrence of oxidative bursts. O2 concentration also fluctuated (Fig. 3a), which might be caused by the scavenging activity of catalase, which converts H2O2 to O2 and water. However, no quantitative assessment of the correlation between the H2O2 and O2 fluctuations can be undertaken, since the microsensors were not positioned directly on the same polyp but on neighboring polyps.
Previous attempts to define extracellular ROS dynamics on live corals quantified ROS in the ambient water sampled from nearby coral tissue (Saragosti et al. 2010; Armoza-Zvuloni and Shaked 2014; Armoza-Zvuloni et al. 2016; Diaz et al. 2016; Szabó et al. 2020). These measurements fail to reflect the temporal dynamics and variability of ROS, highlighting the importance of fine-scale temporal and spatial resolution measurements at the coral tissue interface to capture ROS dynamics within the coral holobiont (Ousley et al. 2022). Despite the limitations imposed by the exclusive measurement of H2O2 (and NO due to the cross-sensitivity of the microsensor) in our study, we contend that H2O2 remains one of the most important ROS in the process of bleaching (Downs et al. 2002). Furthermore, our research enabled a highly time-resolved monitoring of both H2O2, and O2 concentrations, yielding novel insights into the chronological sequence of events unfolding through thermal stress. For future studies, we propose to investigate the production of other ROS during thermal stress using time-resolved monitoring methods.
High concentrations of ROS are commonly associated with thermal stress (Lesser 2011). Specifically, the increased production of H2O2 in algal endosymbionts at elevated temperatures has often been reported (Lesser et al. 1990; Lesser 1996; Smith et al. 2005). However, we show that this relationship is not always observed and becomes only apparent at high levels of heat stress (39 °C). Also, the relationship is more complex due to the multifaceted sources of H2O2 including oxidative bursts, which naturally occur in healthy corals (Ousley et al. 2022) (Fig. 3a). Previous studies detected decreased photosynthetic efficiency and increased oxidative stress during elevated temperatures in algal symbiont cultures (Lesser 1996) and corals (Lesser 1997; Downs et al. 2002; Saragosti et al. 2010). Counter to our initial expectation, moderate heat stress (36 °C) that triggered bleaching did not result in higher H2O2 concentrations compared to measurements obtained at the baseline temperature and under minor heat stress (Figs. 3a, S11). Importantly, we observed all hallmark signs of bleaching (i.e., decreased O2 concentrations during the day, reduced photosynthetic efficiency, coral pigmentation loss, and reduction in Symbiodiniaceae cell numbers) in corals exposed to moderate heat stress (36 °C) (Figs. 3a, b). Thus, our findings indicate that net H2O2 production is not directly related to (i.e., is decoupled from) photodamage and support previous studies that suggest that H2O2 is not the initial trigger of bleaching (Tolleter et al. 2013; Rädecker et al. 2021; Dungan et al. 2022). Although we cannot exclude that the bleaching response was accompanied by increases in the intracellular gross production rate of H2O2, we could not measure an increase in net release of H2O2 at the polyp surface. Since H2O2 molecules can cross polyp membranes, it can be assumed that gross H2O2 production did not exceed the intracellular capacity of antioxidant scavenging. Unexpectedly, we observed an overall decrease in H2O2 release in corals exposed to moderate heat stress but not in corals held at the baseline temperature or under minor heat stress. This may be explained by reduced gross H2O2 production or enhanced antioxidant activity within the coral holobiont (Figs. 3a, S11). Considering the role of H2O2 in cell signaling and the establishment of symbiotic relationships (Marino 2012; Rosset et al. 2021), the paucity of H2O2 may (initially) underlie the loss of Symbiodiniaceae (Rädecker et al. 2021).
Importantly, during high heat stress (39 °C), a different type of H2O2 production was observed after O2 concentration declined and the temperature surpassed 36 °C during the ramp-up phase (Figs. 3a, 4). Notably, both the decreased occurrence of oxidative bursts and the steady increase of H2O2 concentration point toward the breakdown of H2O2 homeostasis of the coral holobiont. A plausible explanation for this observation would be the controlled extracellular production of H2O2 via NADPH oxidase during bursting in contrast to an uncontrolled intracellular source during the steady increase, potentially caused by tissue necrosis and coral mortality. This is supported by the observation that neither switching the light on nor off impacted the already increasing or decreasing trajectories in H2O2 concentrations. Furthermore, holding the temperature at 39 °C for an additional hour and increasing it to 39 °C for a second time after returning to the baseline temperature did not provoke changes or a second increase in H2O2 concentration (Fig. 4b). Interestingly, the H2O2 concentration maxima reached during high heat stress were lower than the short burst maxima observed at the baseline temperature and under minor heat stress, yet resulted in the complete loss of photosynthetic activity. Although high heat stress can increase H2O2 production, it can also negatively influence the antioxidant capacity of the coral holobiont and therefore cause the steady increase in H2O2 concentration (Weis 2008). However, our study cannot differentiate between an increased production of H2O2 from the source and a decrease in the capacity of the coral holobiont to act as an H2O2 sink. Nevertheless, our findings confirm that multiple sources of H2O2 coexist within the coral holobiont and determine net H2O2 levels.
Photosynthesis-independent increase in H2O2 concentration
Damage to the photosystem of algal symbionts due to thermal stress has often been proposed as a major source of ROS (Lesser 1997; Downs et al. 2002; Smith et al. 2005). However, tracking H2O2 concentrations at night under high heat stress (39 °C), we show that the increasing H2O2 concentration is light-independent. H2O2 concentration was already increasing steeply before switching the light on at 07.00h, and this increase was unaffected by the light condition (on or off) (Fig. 4, Figs. S13–S18). These findings show that H2O2 concentration is decoupled from photosynthesis and even from photosynthetically derived O2 (Fig. 4b). Therefore, our results show that photosynthesis within the algal symbiont is not the major source of increasing H2O2 concentrations during high heat stress, arguing for a source of H2O2 within the coral holobiont.
Given that thermal stress increases oxidative stress in algal cultures (Lesser 1996), previous ROS studies have often focused on the photosynthetic symbiont. However, coral bleaching can occur in the absence of light (Tolleter et al. 2013). Furthermore, ROS is produced in bleached corals (Saragosti et al. 2010) and when the electron flow of the photosystem machinery is chemically inhibited (Ousley et al. 2022), pointing at other ROS sources within the coral holobiont. For instance, thermal stress can increase respiration and electron transport inhibition of the coral host and algal symbionts within the mitochondria as well as of associated bacteria, resulting in the increased production of H2O2 (Fridovich 1998; Murphy 2009). Furthermore, sources of NO production within the coral holobiont must also be considered, since the H2O2 microsensor has a cross-sensitivity to NO (Ousley et al. 2022). NO can react with superoxide and form highly reactive hydroxyl radicals and inactivate the mitochondrial electron transport chain, further increasing ROS concentrations (Radi et al. 2000). Notably, NO concentration increases under elevated temperatures and is also likely involved in coral bleaching (Perez and Weis 2006; Bouchard and Yamasaki 2008; Jury 2022). Lastly, a change in the sink mechanisms may also contribute to a net increase of H2O2 under high heat stress. Within the coral holobiont, H2O2 is highly controlled by antioxidant enzymes that are specialized in scavenging O2− and H2O2 (Fridovich 1998; Mittler 2002). When antioxidant activity decreases, the balance between production and degradation can be disrupted, causing increased H2O2 concentrations.
Disentangling the effect of temperature and light in H2O2 production during coral bleaching is not straightforward especially because both heat and light co-occur in natural reef settings. Our study helps to disentangle the effects of temperature and light on H2O2 production via time-resolved monitoring of H2O2 and O2 concentrations throughout the different temperature profiles of the CBASS. The comparison of H2O2 trajectories throughout the different CBASS profiles allowed to clearly identify the isolated effects of light and temperature (Fig. S12). Furthermore, fine-scale microsensor data allowed the examination of H2O2 concentrations seconds before and after the light is switched on/off to determine whether the trajectory of H2O2 concentrations under heat stress is shifted by the presence or absence of light (Figs. S13-S18). The interactive effect of temperature and light during bleaching in natural field conditions, however, deserves further attention in future studies. In our experimental design, for instance, lights were simply switched on and off to examine the light dependency of the H2O2 production. Light enhances the impact of thermal stress in corals (Warner et al. 1999). Thus, understanding the effect of gradual increases/decreases in light intensity and different light levels on photosynthesis and H2O2 production remains of great value.
We demonstrate that in corals exposed to high heat stress, O2 concentration decreases before H2O2 concentration increases (Fig. 4a). When corals are exposed to elevated temperatures, a decrease in O2 concentration can be initiated by enhanced respiration rates of the host which accelerate O2 consumption (Coles and Jokiel 1977). Furthermore, the loss of algal symbionts as well as the inhibition of photosynthesis due to the reduction of electron transport and damage to the photosystem II could further contribute to the decrease of homeostasis (Long et al. 1994; Niyogi 1999). No net O2 release was detectable after the increase in H2O2 concentration following exposure to high heat stress (Fig. 4). The absence of O2, the decline in photosynthetic efficiency, the loss of coral pigmentation and Symbiodiniaceae cells, and the observed tissue sloughing point to the continuous deterioration of the photosynthetic system, the complete breakdown of symbiosis and coral mortality. By nature of experimental limitations, we could only count Symbiodiniaceae cells following heat stress exposure. Thus, we could not determine the chronological sequence of algal symbiont loss and O2 decline. Further experiments with algal symbiont counts at different time points during heat stress should clarify this.
H2O2 – neither the trigger nor the “smoking gun” but a hallmark of coral bleaching
Coral bleaching occurs in the absence of oxidative stress, indicating ROS-independent bleaching (Diaz et al. 2016; Nielsen et al. 2018). Furthermore, no increase in ROS concentration was detected in the coral model Exaipasia diaphana although photosynthetic efficiency decreased after 12 days of thermal stress exposure reaching 34 °C (Dungan et al. 2022). Other studies mark the occurrence of coral bleaching before an increase in antioxidant activity is noted, suggesting the presence of oxidative stress at a later stage (Gardner et al. 2017). These studies highlight the importance of disentangling the chronological sequence of ROS production and coral bleaching and support our findings of H2O2-independent bleaching. This view may be challenged by the ability of exogenous antioxidants to mitigate bleaching (Lesser 1997). However, we also provide evidence of H2O2-induced loss of photosynthetic activity. We show that H2O2 increases during high heat stress (39 °C) but that this is not connected to the prior decrease in photosynthetic performance, coral pigmentation, or symbiont loss during moderate heat stress (36 °C). While the sustained production of H2O2 during high heat stress seems to correlate well with bleaching, correlation does not imply causation. The key question thus becomes, whether increasing concentrations of H2O2 are the cause of bleaching or the consequence of a bleaching coral holobiont. Based on the temporal resolution of our data, we provide evidence that the continuously increasing concentration of H2O2 during high heat stress is a consequence of the bleaching process. Hence, we conclude that increasing H2O2 concentrations are neither the trigger nor the “smoking gun” of coral bleaching within our experimental framework, but rather a hallmark accompanying the sequence of events underlying coral bleaching. Therefore, our results support growing evidence that coral bleaching is driven by H2O2-independent mechanisms (Morris et al. 2019; Rädecker et al. 2023; Rädecker et al. 2021).
Conclusion
H2O2 is a prevalent ROS in coral holobionts and considered one of the underlying causes of coral bleaching, leading to the loss/expulsion of algal symbionts (Downs et al. 2002). Our study provides evidence that H2O2 is neither the direct (trigger) nor the proximate cause (“smoking gun”) of coral bleaching. Coral holobionts constantly produce H2O2 and oxidative bursts seem to be a hallmark of coral functioning, whereas the steady increase in H2O2 levels under high heat stress indicates the breakdown of controlled H2O2 production within the coral holobiont and eventual coral mortality. We found that photosynthetically produced H2O2 is neither the major source of oxidative bursting nor the cause of increasing H2O2 under high heat stress. Importantly, although corals bleached under moderate heat stress, increasing H2O2 concentrations were only detected under high heat stress. Resolving the chronological sequence of ROS production and coral bleaching holds the key to better understanding the role of ROS within the coral holobiont. Identifying the causative trigger of coral bleaching is important to devise strategies that mitigate bleaching and improve predictions of climate change impacts on coral reefs.
Data availability
The datasets generated and/or analyzed during the current study are available in the Supplement. Raw data and scripts are available in the associated GitHub repository CBASS_Microsensor_ROS at https://github.com/MarlenSchlotheuber/CBASS_Microsensor_ROS.
References
Aguirre J, Ríos-Momberg M, Hewitt D, Hansberg W (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol 3(13):111–118. https://doi.org/10.1016/j.tim.2005.01.007
Alderdice R, Perna G, Cárdenas A, Hume BCC, Wolf M, Kühl M, Pernice M, Suggett DJ, Voolstra CR (2022) Deoxygenation lowers the thermal threshold of coral bleaching. Sci Rep 12(1):18273. https://doi.org/10.1038/s41598-022-22604-3
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Armoza-Zvuloni R, Shaked Y (2014) Release of hydrogen peroxide and antioxidants by the coral Stylophora pistillata to its external milieu. Biogeosciences. https://doi.org/10.5194/bg-11-4587-2014
Armoza-Zvuloni R, Schneider A, Sher D, Shaked Y (2016) Rapid hydrogen peroxide release from the coral Stylophora pistillata during feeding and in response to chemical and physical stimuli. Sci Rep 6(1):1–10. https://doi.org/10.1038/srep21000
Bouchard JN, Yamasaki H (2008) Heat stress stimulates nitric oxide production in Symbiodinium microadriaticum: a possible linkage between nitric oxide and the coral bleaching phenomenon. Plant Cell Physiol 49(4):641–652. https://doi.org/10.1093/pcp/pcn037
Brown BE (1997) Coral bleaching: causes and consequences. Coral Reefs. https://doi.org/10.1007/s003380050249
Byczkowski JZ, Gessner T (1988) Biological role of superoxide ion-radical. Int J Biochem 20(6):569–580. https://doi.org/10.1016/0020-711X(88)90095-X
Coles SL, Jokiel PL (1992) Effects of salinity on coral reefs. In: Pollution in tropical aquatic systems. CRC Press, pp 147–166
Coles SL, Jokiel PL (1977) Effects of temperature on photosynthesis and respiration in hermatypic corals. Mar Biol 43(3):209–216. https://doi.org/10.1007/BF00402313
Cornwall CE, Comeau S, Kornder NA, Perry CT, van Hooidonk R, DeCarlo TM, Pratchett MS, Anderson KD, Browne N, Carpenter R, Diaz-Pulido G, Fortunato SAV, Kennedy E, Lantz CA, McCulloch MT, González-Rivero M, Schoepf V, Smithers SG, Lowe RJ (2021) Global declines in coral reef calcium carbonate production under ocean acidification and warming. PNAS. https://doi.org/10.1073/pnas.2015265118
Costanza R, de Groot R, Sutton P, van der Ploeg S, Anderson SJ, Kubiszewski I, Farber S, and Turner RK (2014) Changes in the Global Value of Ecosystem Services. Global Environmental Change 26 (5):152–58. https://doi.org/10.1016/j.gloenvcha.2014.04.002
Cronenberg C, van Groen B, de Beer D, van den Heuvel H (1991) Oxygen-independent glucose microsensor based on glucose oxidase. Anal Chim Acta 242:275–278. https://doi.org/10.1016/0003-2670(91)87075-I
Daniels CA, Zeifman A, Heym K, Ritchie KB, Watson CA, Berzins I, Breitbart M (2011) Spatial heterogeneity of bacterial communities in the mucus of Montastraea annularis. Mar Ecol Prog Ser 426:29–40. https://doi.org/10.3354/meps09024
Diaz JM, Hansel CM, Apprill A, Brighi C, Zhang T, Weber L, McNally S, Xun L (2016) Species-specific control of external superoxide levels by the coral holobiont during a natural bleaching event. Nat Commun 7(1):1–10. https://doi.org/10.1038/ncomms13801
Dixon GB, Davies SW, Aglyamova GV, Meyer E, Bay LK, Matz MV (2015) Genomic determinants of coral heat tolerance across latitudes. Science 348(6242):1460–1462. https://doi.org/10.1126/science.1261224
Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM (2002) Oxidative stress and seasonal coral bleaching. Free Radical Biol Med 33(4):533–543. https://doi.org/10.1016/S0891-5849(02)00907-3
Dungan AM, Maire J, Perez-Gonzalez A, Blackall LL, van Oppen MJ (2022) Lack of evidence for the oxidative stress theory of bleaching in the sea anemone, Exaiptasia diaphana, under elevated temperature. Coral Reefs. https://doi.org/10.1007/s00338-022-02251-w
Dunn S, Thomason J, Tissier ML, Bythell J (2004) Heat stress induces different forms of cell death in sea anemones and their endosymbiotic algae depending on temperature and duration. Cell Death Differ. https://doi.org/10.1038/sj.cdd.4401484
Eakin CM, Devotta D, Heron SF, Connolly S, Liu G, Geiger E, Cour JDL, Gomez A, Skirving W, Baird AH, Cantin N, Couch C, Donner S, Gilmour J, Gonzalez-Rivero M, Gudka M, Harrison H, Hodgson G, Hoegh-Guldberg O,...Manzello D (2022) The 2014–17 global coral bleaching event: the most severe and widespread coral reef destruction. Res Square. https://doi.org/10.21203/rs.3.rs-1555992/v1
Evensen NR, Fine M, Perna G, Voolstra CR, Barshis DJ (2021) Remarkably high and consistent tolerance of a Red Sea coral to acute and chronic thermal stress exposures. Limnol Oceanogr 66(5):1718–1729. https://doi.org/10.1002/lno.11715
Evensen NR, Parker KE, Oliver TA, Palumbi SR, Logan CA, Ryan JS, Klepac CN, Perna G, Warner ME, Voolstra CR, Barshis DJ (2023) The Coral Bleaching Automated Stress System (CBASS): A low‐cost portable system for standardized empirical assessments of coral thermal limits. Abstr Lim Oceanography: Methods 21(7):421–434. https://doi.org/10.1002/lom3.v21.710.1002/lom3.10555
Falkowski PG, Dubinsky Z, Santostefano G (1985) Light-enhanced dark respiration in phytoplankton. Book. https://doi.org/10.1080/03680770.1983.11897784
Fridovich I (1998) Oxygen toxicity: a radical explanation. J Exp Biol 201(8):1203–1209. https://doi.org/10.1242/jeb.201.8.1203
Gardner SG, Raina J-B, Ralph PJ, Petrou K (2017) Reactive oxygen species (ROS) and dimethylated sulphur compounds in coral explants under acute thermal stress. J Exp Biol. https://doi.org/10.1242/jeb.153049
Gates RD, Baghdasarian G, Muscatine L (1992) Temperature stress causes host cell detachment in symbiotic cnidarians: implications for coral bleaching. Bio Bull. https://doi.org/10.2307/1542252
Glynn PW (1993) Coral reef bleaching: ecological perspectives. Coral Reefs 12(1):1–17. https://doi.org/10.1007/BF00303779
Hansel CM, Diaz JM (2021) Production of extracellular reactive oxygen species by marine biota. Ann Rev Mar Sci 13:177–200. https://doi.org/10.1146/annurev-marine-041320-102550
Hawkins TD, Bradley BJ, Davy SK (2013) Nitric oxide mediates coral bleaching through an apoptotic-like cell death pathway: evidence from a model sea anemone-dinoflagellate symbiosis. FASEB J. https://doi.org/10.1093/pcp/pcs127
Hopkins R (2016) Superoxide in biology and medicine: an overview. React Oxygen Species. https://doi.org/10.20455/ros.2016.825
Hughes DJ, Alderdice R, Cooney C, Kühl M, Pernice M, Voolstra CR & Suggett DJ (2020) Coral reef survival under accelerating ocean deoxygenation. Nat Clim Change 10(4):296–307. https://doi.org/10.1038/s41558-020-0737-9
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R (2017) Global warming and recurrent mass bleaching of corals. Nature 543(7645):373–377. https://doi.org/10.1038/nature21707
Jury CP, Boeing BM, Trapido-Rosenthal H, Gates RD, and Toonen RJ (2022) Nitric oxide production rather than oxidative stress and cell death is associated with the onset of coral bleaching in pocillopora acuta. PeerJ. https://doi.org/10.7717/peerj.13321
Krueger T, Hawkins TD, Becker S, Pontasch S, Dove S, Hoegh-Guldberg O, Leggat W, Fisher PL, Davy SK (2015) Differential coral bleaching—contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comp Biochem Physiol A Mol Integr Physiol 190:15–25. https://doi.org/10.1016/j.cbpa.2015.08.012
Lesser MP (1996) Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol Oceanogr. https://doi.org/10.4319/lo.1996.41.2.0271
Lesser MP (1997) Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16(3):187–192. https://doi.org/10.1007/s003380050073
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278. https://doi.org/10.1146/annurev.physiol.68.040104.110001
Lesser MP, Stochaj WR, Tapley DW, Shick JM (1990) Bleaching in coral reef anthozoans: effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 8(4):225–232. https://doi.org/10.1007/BF00265015
Lesser MP (2011) Coral bleaching: causes and mechanisms. In: Coral reefs: an ecosystem in transition. Springer, pp 405–419
Lesser MP, Farrell JH (2004) Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23(3):367–377. https://doi.org/10.1007/s00338-004-0392-z
Libro S, Kaluziak ST, Vollmer SV (2013) RNA-seq profiles of immune related genes in the staghorn coral Acropora cervicornis infected with white band disease. PLoS ONE. https://doi.org/10.1371/journal.pone.0081821
Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45:633–662. https://doi.org/10.1146/annurev.pp.45.060194.003221
Marino D, Marino D, Dunand C, Puppo A, and Pauly N (2012) A burst of plant NADPH oxidases. Cell Press. https://doi.org/10.1016/j.tplants.2011.10.001
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Morikawa MK, Palumbi SR (2019) Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc Natl Acad Sci 116(21):10586–10591. https://doi.org/10.1073/pnas.1721415116
Morris LA, Voolstra CR, Quigley KM, Bourne DG, Bay LK (2019) Nutrient availability and metabolism affect the stability of coral-Symbiodiniaceae symbioses. Trends Microbiol 27(8):678–689. https://doi.org/10.1016/j.tim.2019.03.004
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem Soc. https://doi.org/10.1042/BJ20081386
Naumann MS, Niggl W, Laforsch C, Glaser C, Wild C (2009) Coral surface area quantification–evaluation of established techniques by comparison with computer tomography. Coral Reefs. https://doi.org/10.1007/s00338-008-0459-3
Nielsen DA, Petrou K, Gates RD (2018) Coral bleaching from a single cell perspective. ISME J 12(6):1558–1567. https://doi.org/10.1038/s41396-018-0080-6
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Biol 50(1):333–359. https://doi.org/10.1146/annurev.arplant.50.1.333
Ousley S, de Beer D, Bejarano S, Chennu A (2022) High-Resolution dynamics of hydrogen peroxide on the surface of Scleractinian corals in relation to photosynthesis and feeding. Front Mar Sci. https://doi.org/10.3389/fmars.2022.812839
Perez S, Weis V (2006) Nitric oxide and cnidarian bleaching: an eviction notice mediates breakdown of a symbiosis. J Exp Biol 209(14):2804–2810. https://doi.org/10.1242/jeb.02309
Pogoreutz C, Rädecker N, Cardenas A, Gärdes A, Voolstra CR, Wild C (2017) Sugar enrichment provides evidence for a role of nitrogen fixation in coral bleaching. Glob Change Biol 23(9):3838–3848. https://doi.org/10.1111/gcb.13695
Rädecker N, Pogoreutz C, Gegner HM, Cárdenas A, Roth F, Bougoure J, Guagliardo P, Wild C, Pernice M, Raina J-B, Meibom A, Voolstra CR (2021) Heat stress destabilizes symbiotic nutrient cycling in corals. Proc Natl Acad Sci 118(5):e2022653118. https://doi.org/10.1073/pnas.2022653118
Rädecker N, Escrig S, Spangenberg JE, Voolstra CR, Meibom A (2023) Coupled carbon and nitrogen cycling regulates the cnidarian–algal symbiosis. Abstr Nat Commun 14(1). https://doi.org/10.1038/s41467-023-42579-7
Radi R, Denicola A, Alvarez B, Ferrer-Sueta G, Rubbo H (2000) The biological chemistry of peroxynitrite. In: Nitric oxide. Elsevier, pp 57–82. https://doi.org/10.1016/B978-012370420-7/50005-8
Revsbech NP, Jørgensen BB (1986) Microelectrodes: their use in microbial ecology. In: Marshall KC (ed) Advances in microbial ecology. Springer, New York, pp 293–352. https://doi.org/10.1007/978-1-4757-0611-6_7
Ritz C, Streibig JC (2005) Bioassay analysis using R. J Stat Softw 12:1–22. https://doi.org/10.1371/journal.pone.0146021
Richier S, Furla P, Plantivaux A, Merle PL, Allemand D (2005) Symbiosis-induced adaptation to oxidative stress. J Exp Biol 208 (Pt 2):277–285. https://doi.org/10.1242/jeb.01368
Roberty S, Fransolet D, Cardol P, Plumier J-C, Franck F (2015) Imbalance between oxygen photoreduction and antioxidant capacities in Symbiodinium cells exposed to combined heat and high light stress. Coral Reefs 34(4):1063–1073. https://doi.org/10.1007/s00338-015-1328-5
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10. https://doi.org/10.3354/meps243001
Rosset SL, Oakley CA, Ferrier-Pagès C, Suggett DJ, Weis VM, Davy SK (2021) The molecular language of the cnidarian-dinoflagellate symbiosis. Trends Microbiol 29(4):320–333. https://doi.org/10.1016/j.tim.2020.08.005
Röthig T, Trevathan‐Tackett SM, Voolstra CR, Ross C, Chaffron S, Durack PJ, Warmuth LM, Sweet M (2023) Human-induced salinity changes impact marine organisms and ecosystems. Abstr Glob Change Biol 29(17):4731–4749. https://doi.org/10.1111/gcb.v29.1710.1111/gcb.16859
Saragosti E, Tchernov D, Katsir A, Shaked Y (2010) Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium. PLoS ONE 5(9):e12508. https://doi.org/10.1371/journal.pone.0012508
Smith DJ, Suggett DJ, Baker NR (2005) Is photoinhibition of zooxanthellae photosynthesis the primary cause of thermal bleaching in corals? Glob Change Biol 11(1):1–11. https://doi.org/10.1111/j.1365-2486.2004.00895.x
Solayan A (2016) Biomonitoring of coral bleaching—a glimpse on biomarkers for the early detection of oxidative damages in corals. In: Larramendy M, Soloneski S (eds) Invertebrates experimental models in toxicity screening. InTech. https://doi.org/10.5772/61831
Suggett DJ, Smith DJ (2020) Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob Change Biol 26(1):68–79. https://doi.org/10.1111/gcb.14871
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14(6):691–699. https://doi.org/10.1016/j.pbi.2011.07.014
Szabó M, Larkum AW, Vass I (2020) A review: the role of reactive oxygen species in mass coral bleaching. Photosynth Algae Biochem Physiol Mech. https://doi.org/10.1007/978-3-030-33397-3_17
Tchernov D, Gorbunov MY, de Vargas C, Narayan Yadav S, Milligan AJ, Häggblom M, Falkowski PG (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci 101(37):13531–13535. https://doi.org/10.1073/pnas.0402907101
Tchernov D, Kvitt H, Haramaty L, Bibby TS, Gorbunov MY, Rosenfeld H, Falkowski PG (2011) Apoptosis and the selective survival of host animals following thermal bleaching in zooxanthellate corals. Proc Natl Acad Sci 108(24):9905–9909. https://doi.org/10.1073/pnas.1106924108
Tolleter D, Seneca FO, DeNofrio JC, Krediet CJ, Palumbi SR, Pringle JR, Grossman AR (2013) Coral bleaching independent of photosynthetic activity. Curr Biol 23(18):1782–1786. https://doi.org/10.1016/j.cub.2013.07.041
Van Woesik R, Shlesinger T, Grottoli AG, Toonen RJ, Thurber RV, Warner ME, Hulver AM, Chapron L, McLachlan RH, Albright R, Crandall E, DeCarlo TM, Donovan MK, Eirin J, Harrison HB, Heron SF, Huang D, Humanes A, Krueger T, Zaneveld J (2022) Coral-bleaching responses to climate change across biological scales. Global Change Biol. https://doi.org/10.1111/gcb.16192
Voolstra CR, Buitrago-López C, Perna G, Cárdenas A, Hume BC, Rädecker N, Barshis DJ (2020) Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob Change Biol 26(8):4328–4343. https://doi.org/10.1111/gcb.15148
Voolstra CR, Valenzuela JJ, Turkarslan S, Cárdenas A, Hume BCC, Perna G, Buitrago C, Rowe K, Orellana MV, Baliga NS, Paranjape S, Banc G, Bellworthy J, Fine M, Frias S, Barshis DJ (2021) Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol Ecol. https://doi.org/10.1111/mec.16064
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.96.14.8007
Weis VM (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211(19):3059–3066. https://doi.org/10.1242/jeb.009597
Zamoum T, Furla P (2012) Symbiodinium isolation by NaOH treatment. J Exp Biol. https://doi.org/10.1242/jeb.074955
Acknowledgements
We are grateful for the excellent support of the MPI electrical workshop, the MPI Microsensor group, the MAREE and Biolab teams. Special thanks to Reef Systems’ students Alejandro Plazas Gómez, Anjleen Hannak, Ellis Weinberg, and Karen Kuek for supporting after-hour laboratory work at the ZMT. We also would like to thank Royal Burgers` Zoo for providing P. damicornis coral nubbins. We thank the anonymous reviewers for their critical feedback that helped improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the ZMT and the University of Konstanz. MS is supported by the German Research Foundation (DFG), project 15951622. EFC is supported by the University of Technology Chancellor’s Postdoctoral Research Fellowship and ARC Discovery Early Career Research Award (DE190100142).
Author information
Authors and Affiliations
Contributions
SB, MKS, CRV, DdB, EC, and JK contributed to conceptualization; CRV, DdB, and MKS provided methodology; MKS performed investigation; MKS, MG, and CRV performed data curation; MKS, CRV, and SB carried out formal analysis; MG and MKS performed visualization; MKS, SB, CRV, and DdB performed writing—original draft; MKS, CRV, SB, DdB, EC, JK, KN, and SO performed writing—review and editing; DdB, SB, and CRV provided resources; SB contributed to funding acquisition; SB, CRV, and DdB performed supervision; SB performed project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlotheuber, M., Voolstra, C.R., de Beer, D. et al. High temporal resolution of hydrogen peroxide (H2O2) dynamics during heat stress does not support a causative role in coral bleaching. Coral Reefs 43, 119–133 (2024). https://doi.org/10.1007/s00338-023-02448-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02448-7