Abstract
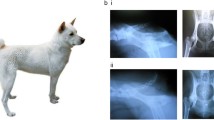
Most mammals possess a tail, humans and the Great Apes being notable exceptions. One approach to understanding the mechanisms and evolutionary forces influencing development of a tail is to identify the genetic factors that influence extreme tail length variation within a species. In mice, the Tailless locus has proven to be complex, with evidence of multiple different genes and mutations with pleiotropic effects on tail length, fertility, embryogenesis, male transmission ratio, and meiotic recombination. Five cat breeds have abnormal tail length phenotypes: the American Bobtail, the Manx, the Pixie-Bob, the Kurilian Bobtail, and the Japanese Bobtail. We sequenced the T gene in several independent lineages of Manx cats from both the US and the Isle of Man and identified three 1-bp deletions and one duplication/deletion, each predicted to cause a frameshift that leads to premature termination and truncation of the carboxy terminal end of the Brachyury protein. Ninety-five percent of Manx cats with short-tail phenotypes were heterozygous for T mutations, mutant alleles appeared to be largely lineage-specific, and a maximum LOD score of 6.21 with T was obtained at a recombination fraction (Θ) of 0.00. One mutant T allele was shared with American Bobtails and Pixie-Bobs; both breeds developed more recently in the US. The ability of mutant Brachyury protein to activate transcription of a downstream target was substantially lower than wild-type protein. Collectively, these results suggest that haploinsufficiency of Brachyury is one mechanism underlying variable tail length in domesticated cats.




Similar content being viewed by others
References
Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101
Basrur PK, Deforest ME (1979) Embryological impact of the Manx gene. Carnivore Genet Newslett 3:378–384
Conlon FL, Sedgwick SG, Weston KM, Smith JC (1996) Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development 122:2427–2435
Darwin C (1868) The variation of animals and plants under domestication. John Murray, London
Deforest ME, Basrur PK (1979) Malformations and the Manx syndrome in cats. Can Vet J 20:304–314
Green ST, Green FA (1987) The Manx cat: an animal model for neural tube defects. Mater Med Pol 19:219–221
Haworth K, Putt W, Cattanach B, Breen M, Binns M, Lingaas F, Edwards YH (2001) Canine homolog of the T-box transcription factor T; failure of the protein to bind to its DNA target leads to a short-tail phenotype. Mamm Genome 12:212–218
Howell JM, Siegel PB (1963) Phenotypic variability of taillessness in Manx cats. J Hered 54:167–169
Howell JM, Siegel PB (1966) Morphological effects of the Manx factor in cats. J Hered 57:100–104
Hytonen MK, Grall A, Hedan B, Dreano S, Seguin SJ, Delattre D, Thomas A, Galibert F, Paulin L, Lohi H, Sainio K, Andre C (2009) Ancestral T-box mutation is present in many, but not all, short-tailed dog breeds. J Hered 100:236–240
Indrebo A, Langeland M, Juul HM, Skogmo HK, Rengmark AH, Lingaas F (2008) A study of inherited short tail and taillessness in Pembroke Welsh corgi. J Small Anim Pract 49:220–224
James CC, Lassman LP, Tomlinson BE (1969) Congenital anomalies of the lower spine and spinal cord in Manx cats. J Pathol 97:269–276
Kispert A, Koschorz B, Herrmann BG (1995) The T protein encoded by Brachyury is a tissue-specific transcription factor. EMBO J 14:4763–4772
Kitchen H, Murray RE, Cockrell BY (1972) Animal model for human disease. Spina bifida, sacral dysgenesis and myelocele. Animal model: Manx cats. Am J Pathol 68:203–206
Kurushima JD, Lipinski MJ, Gandolfi B, Froenicke L, Grahn JC, Grahn RA, Lyons LA (2013) Variation of cats under domestication: genetic assignment of domestic cats to breeds and worldwide random-bred populations. Anim Genet 44(3):311–324
Leipold HW, Huston K, Blauch B, Guffy MM (1974) Congenital defects on the caudal vertebral column and spinal cord in Manx cats. J Am Vet Med Assoc 164:520–523
Lipinski MJ, Froenicke L, Baysac KC, Billings NC, Leutenegger CM, Levy AM, Longeri M, Niini T, Ozpinar H, Slater MR, Pedersen NC, Lyons LA (2008) The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics 91:12–21
Martin AH (1971) A congenital defect in the spinal cord of the Manx cat. Vet Pathol 8:232–238
Orpana AK, Ho TH, Stenman J (2012) Multiple heat pulses during PCR extension enabling amplification of GC-rich sequences and reducing amplification bias. Anal Chem 84:2081–2087
Plummer SB, Bunch SE, Khoo LH, Spaulding KA, Kornegay JN (1993) Tethered spinal cord and an intradural lipoma associated with a meningocele in a Manx-type cat. J Am Vet Med Assoc 203:1159–1161
Robinson R (1993) Expressivity of the Manx gene in cats. J Hered 84:170–172
Schulte-Merker S, van Eeden FJ, Halpern ME, Kimmel CB, Nusslein-Volhard C (1994) No tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120:1009–1015
Todd NB (1963) Independent assortment of Manx and three coat color mutants in domestic cat. J Hered 54:266
Todd NB (1964) The Manx factor in domestic cats. A possible genetic basis for expressivity of taillessness and other associated anomalies. J Hered 55:225–230
Wu B, Shao Y, Chen B, Liu C, Xue Z, Wu P, Li H (2010) Identification of a novel mouse brachyury (T) allele causing a short tail mutation in mice. Cell Biochem Biophys 58:129–135
Acknowledgments
We are deeply indebted to the community of Manx, American Bobtail, and Japanese Bobtail breeders and the Mann Cat Sanctuary for their assistance and helpful discussion. We also thank Bill Clark and Dawn Reding of Iowa State University for American Bobcat DNA samples. Funding was provided in part by the National Center for Research Resources (R24 RR016094) and research is currently supported by the Office of Research Infrastructure Programs (OD R24OD010928), the Winn Feline Foundation (grant 10-015), and the UC Davis Center for Companion Animal Health George and Phyllis Miller Feline Health Fund (LAL). Research was also supported by grants from the NIAID (AI061061) and the American Cancer Society (RSG-09-045-01-DDC) to ASW.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
335_2013_9471_MOESM1_ESM.pdf
Supplementary Table 1. Primers used to amplify and sequence coding regions of the feline T gene. Primers with “PCR” in the primer name were used for amplification and primers with “Seq” in the primer name were used for sequencing. Primers marked with $ and # indicate primers with identical sequence that were used in PCR and sequencing, respectively. All primers map to introns and capture the complete coding sequence of the specified exon and the intron–exon boundaries, with the exception of the forward primer for exon 2 (noted with an *), which is exonic and captures only a portion of the coding sequence (PDF 30 kb)
335_2013_9471_MOESM2_ESM.pdf
Supplementary Table 2. Sequences and map positions (Sep 2011 ICGSC Felis_catus 6.2/ felCat5) of the coding exons of the feline T gene. A partial sequence was deposited in the European Nucleotide Archive under accession number HG004538 (http://www.ebi.ac.uk/ena/data/view/HG004538) (PDF 34 kb)
335_2013_9471_MOESM3_ESM.pdf
Supplementary Table 3. Description of tail lengths and mutations in Manx cats with short-tailed cats categorized by specific tail length. (PDF 27 kb)
335_2013_9471_MOESM6_ESM.eps
Supplementary Fig. 1 Delineation of sub-pedigrees used in linkage analysis. To facilitate linkage analysis, the complex pedigrees were divided into six smaller subpedigrees defined by various line colors: Pedigree A1 containing subpedigree 1 (orange), 2 (navy blue), 3 (green), and 4 (pink); Pedigree A1 and A2 containing subpedigree 5 (purple); and Pedigree B containing subpedigree 6 (teal). Individuals connected with black lines were not used in linkage analysis. (EPS 626 kb)
335_2013_9471_MOESM7_ESM.eps
Supplementary Fig. 2. Manx Pedigree B. Extended pedigree illustrating relationships among Manx cats used for linkage and mutation analysis. Squares represent male cats and circles represent female cats. Cats with reduced tail lengths are denoted by varied darkened symbols: rumpy, quarter filled; rumpy-riser, half filled; stumpy, three-quarter filled. Full-tailed cats are indicated with open symbols and patterned symbols denote individuals for whom no phenotypic data were available. Males represented more than once in the pedigree are indicated with a double square labeled with the generation and individual number of the first time they appear in the pedigree. Genotypes for the c.998delT and c.1169delC or c.1199delC T mutants are indicated below each symbol. A “-” indicates the mutation was absent. (EPS 411 kb)
Rights and permissions
About this article
Cite this article
Buckingham, K.J., McMillin, M.J., Brassil, M.M. et al. Multiple mutant T alleles cause haploinsufficiency of Brachyury and short tails in Manx cats. Mamm Genome 24, 400–408 (2013). https://doi.org/10.1007/s00335-013-9471-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-013-9471-1