Abstract
Background
Programmed death-ligand 1 (PD-L1) expression is a predictive biomarker for immunotherapy in non-small cell lung cancer (NSCLC). PD-L1 and glucose transporter 1 expression are closely associated, and studies demonstrate correlation of PD-L1 with glucose metabolism.
Aim
The aim of this study was to investigate the association of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG-PET/CT) metabolic parameters with PD-L1 expression in primary lung tumour and lymph node metastases in resected NSCLC.
Methods
We conducted a retrospective analysis of 210 patients with node-positive resectable stage IIB–IIIB NSCLC. PD-L1 tumour proportion score (TPS) was determined using the DAKO 22C3 immunohistochemical assay. Semi-automated techniques were used to analyse pre-operative [18F]FDG-PET/CT images to determine primary and nodal metabolic parameter scores (including max, mean, peak and peak adjusted for lean body mass standardised uptake values (SUV), metabolic tumour volume (MTV), total lesional glycolysis (TLG) and SUV heterogeneity index (HISUV)).
Results
Patients were predominantly male (57%), median age 70 years with non-squamous NSCLC (68%). A majority had negative primary tumour PD-L1 (TPS < 1%; 53%). Mean SUVmax, SUVmean, SUVpeak and SULpeak values were significantly higher (p < 0.05) in those with TPS ≥ 1% in primary tumour (n = 210) or lymph nodes (n = 91). However, ROC analysis demonstrated only moderate separability at the 1% PD-L1 TPS threshold (AUCs 0.58–0.73). There was no association of MTV, TLG and HISUV with PD-L1 TPS.
Conclusion
This study demonstrated the association of SUV-based [18F]FDG-PET/CT metabolic parameters with PD-L1 expression in primary tumour or lymph node metastasis in resectable NSCLC, but with poor sensitivity and specificity for predicting PD-L1 positivity ≥ 1%.
Clinical relevance statement
Whilst SUV-based fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography metabolic parameters may not predict programmed death-ligand 1 positivity ≥ 1% in the primary tumour and lymph nodes of resectable non-small cell lung cancer independently, there is a clear association which warrants further investigation in prospective studies.
Trial registration
Non-applicable
Key Points
• Programmed death-ligand 1 immunohistochemistry has a predictive role in non-small cell lung cancer immunotherapy; however, it is both heterogenous and dynamic.
• SUV-based fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG-PET/CT) metabolic parameters were significantly higher in primary tumour or lymph node metastases with positive programmed death-ligand 1 expression.
• These SUV-based parameters could potentially play an additive role along with other multi-modal biomarkers in selecting patients within a predictive nomogram.
Similar content being viewed by others
Background
Monoclonal antibodies targeting programmed cell death protein-1 (PD-1) or programmed death-ligand 1 (PD-L1) have revolutionised treatment approaches of several cancers, including non-small cell lung cancer (NSCLC) [1,2,3,4,5]. PD-L1 tumour proportion score (TPS), measured by immunohistochemistry, is a validated biomarker for anti-PD-1/PD-L1 therapies [6, 7]. In advanced NSCLC, a PD-L1 TPS ≥ 50% indicates a likely response to anti-PD-1/PD-L1 therapy, negating the need for cytotoxic combination and its associated toxicities [1]. However, not all PD-L1 expressors respond, temporospatial heterogeneity of expression is well-documented, and there are several PD-L1 assays available; as such, patient selection remains a significant challenge [8, 9].
The neoadjuvant CheckMate 816 trial of nivolumab plus chemotherapy vs chemotherapy alone in early-stage NSCLC demonstrated benefits in pathological complete response rates (24.0% vs 2.2%, respectively) and in median event-free survival (31.6 vs 20.8 months) in stage IB–IIIA NSCLC [10]. The benefit was present across all PD-L1 TPS groups, but event-free survival in those with tumour PD-L1 expression ≥ 1% was improved.
Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG-PET/CT) is an important imaging modality used to stage NSCLC [11]. [18F]FDG accumulation correlates with the expression of glucose transporter 1 (GLUT1), hexokinase II (HK2) and hypoxia-inducible factor 1-alpha (HIF-1α), with roles in glucose metabolism and hypoxia, respectively [12]. Several studies have demonstrated the correlation of [18F]FDG uptake, as well as GLUT1, HK2 and HIF-1α expression, with PD-L1 expression [13,14,15]. It is therefore unsurprising that [18F]FDG-PET/CT metabolic parameters have been associated with PD-L1 immunohistochemistry and/or response to anti-PD-1/PD-L1 therapy [16,17,18,19,20,21,22,23,24].
The majority of reported studies have been conducted in advanced disease and/or focus on PD-L1 expression of the primary lesion only. Our aim, therefore, was to investigate the role of various metabolic parameters, including those of tumour burden, measured using pre-operative [18F]FDG-PET/CT, in predicting PD-L1 expression of primary tumour and nodal metastases in patients with resectable NSCLC.
Methods
The study was approved by UK Research Ethics Committee (UK IRAS 228790) and within the Guy’s Cancer Cohort (ref: 18/NW/0297) [25]. We conducted a retrospective analysis of 495 consecutive patients with node-positive (stage IIB–IIIB, IASLC TNM 8th ed.) and treatment-naïve NSCLC who were referred to and underwent primary resection at a tertiary centre between February 2009 and October 2018 (Fig. 1). Eligibility criteria for the study included age ≥ 18 years, histological diagnosis of NSCLC and pathologically confirmed N1/N2 lymph node involvement. Patients had all been treated with curative-intent surgical resection of the primary lung tumour, and hilar and/or mediastinal lymphadenectomy. Patients were excluded if there was no available or assessable pre-operative [18F]FDG PET/CT imaging within 3 months, clinical data and/or histopathological specimens are not available or they received pre-operative systemic anti-cancer and/or radio-therapy. Electronic medical records were reviewed for demographics including age, smoking history, diagnosis and surgery dates, histology and pathological stage.
Histopathological assessment
Resected specimen haematoxylin and eosin slides were assessed to confirm the histopathological diagnosis and quality/quantity of tumour to select representative formalin-fixed paraffin-embedded tissue blocks from the primary tumour and nodal metastases where present. If more than one lymph node was involved, the node furthest from the primary tumour was used.
PD-L1 immunohistochemistry was performed using the 22C3 pharmDx assay on the Dako Autostainer Link 48 (Agilent Technologies Inc.). A minimum of 100 viable tumour cells were required for analysis; stained slides were assessed by two independent consultant histopathologists [26]. The TPS was calculated as the percentage of PD-L1-positive tumour cells relative to all viable tumour cells in the specimen. Patients were subsequently categorised as TPS < 1%, 1–49% or ≥ 50%, with < or ≥ 1% and < or ≥ 50% thresholds being commonly used for treatment decisions in early-stage and late-stage disease settings [1,2,3,4,5,6,7, 10, 27, 28].
[ 18 F]FDG PET/CT protocol
[18F]FDG PET/CT scans were performed at the local referring centre or at King’s College London & Guy’s and St Thomas’ PET Centre (London, UK) according to local protocols and in accordance with current guidelines [29]. Patients were fasted at least 6 h and glucose levels confirmed < 180 mg/dL prior to imaging. Patients were injected with 2.5–3.5 MBq/kg FDG (median activity 336 MBq [range 194–436]) with images acquired at 60 min post-injection. PET images were reconstructed using iterative techniques and CT for attenuation correction.
Image analysis
Images were reviewed by an experienced nuclear medicine researcher using Hermes GOLD™ (Hermes Medical Solutions). Volumes of interest (VOI), including the primary lung tumour and associated thoracic lymph nodes, were identified with CT correlation (Fig. 2). Malignant lesions (primary and lymph nodes) analysed on imaging were matched with their respective histopathologically assessed lesions, to allow correlation of histological PD-L1 status with metabolic parameters. A semi-automated segmentation method was used to delineate the VOI, with the metabolic tumour volume (MTV, cm3) established using a 40% threshold of the maximum standardised uptake value (SUVmax). The mean SUV (SUVmean), peak SUV (SUVpeak) and SUVpeak adjusted for lean body mass (SULpeak) were also recorded. In addition, primary tumour and thoracic lymph node total lesion glycolysis (TLG = MTV × SUVmean) and SUV-based heterogeneity index (HISUV = SUVmax ÷ SUVmean) were calculated.
Image analysis example. a–f Patient 1 with T1bN2M0 (stage IIIA) non-squamous NSCLC with negative PD-L1 TPS < 1% in both primary and nodal disease. a CT image of primary tumour for anatomical correlation; b PET image of primary tumour depicting VOI with an SUVmax 6.75, SUVmean 4.03, SUVpeak 4.84, SULpeak 3.16, MTV 2.79 cm3, TLG 11.24 and HISUV 1.67; and (c) fused axial PET/CT images showing primary tumour. d CT image of lymph node for anatomical correlation; e PET image of lymph node metastasis depicting VOI with SUVmax 5.19, SUVmean 2.91, SUVpeak 4.05, SULpeak 2.65, MTV 7.65 cm3, TLG 22.29 and HISUV 1.78; f fused axial PET/CT images. g–i Patient 2 with T3N2M0 (stage IIIB) non-squamous NSCLC with positive (high, ≥ 50%) PD-L1 TPS 90% in both primary and nodal metastasis. g CT image of primary tumour and lymph node metastasis for anatomical correlation; h PET image of primary tumour (red arrow) VOI with SUVmax 24.55, SUVmean 12.49, SUVpeak 20.31, SULpeak 19.12, MTV 80.22 cm3, TLG 1002.11 and HISUV 1.97, and lymph node metastasis (yellow arrow) VOI with SUVmax 16.59, SUVmean 12.71, SUVpeak 15.45, SULpeak 13.08, MTV 6.07 cm3, TLG 77.19 and HISUV 1.31; and (i) fused axial PET/CT images. Red arrows denote primary tumour; yellow arrows denote lymph node metastasis. Created with BioRender.com
Statistical analysis
Continuous variables are presented as means with the standard error; categorical variables are presented with absolute and relative frequencies. Categorical data were analysed using the chi-squared test. Normality of continuous variables was assessed using the Shapiro-Wilk test. Correlations between PD-L1 TPS and metabolic parameters were assessed with Spearman’s rank correlation coefficient. Parametric data, according to PD-L1 TPS groups, were analysed using one-way ANOVA, whilst non-parametric data were analysed using the Mann-Whitney U test to compare two groups, or Kruskal-Wallis with Dunn’s multiple comparison test for three or more groups. p values are two-sided with significance as α = 0.05. Youden’s (J) statistic was used to determine the optimal cut-off point from receiver operating characteristic (ROC) curves with equal weight given to sensitivity and specificity. Multiple logistic regression was performed for PD-L1 expression controlling for age, sex, smoking history, histology, tumour (T), nodal (N) stage and SUV-based metabolic parameters of interest. Data were analysed and individual graphs created with GraphPad Prism v9.5.1 for macOS (GraphPad Software). Figures were generated using BioRender.com.
Results
Of 495 consecutive patients, 210 cases met the inclusion criteria; the most common reason for exclusion being PET and/or original DICOM files not available (n = 209) (Fig. 1). Patients’ demographic and clinical characteristics are demonstrated in Table 1, and Supplementary Tables 1–5. Included patients were predominantly male (n = 120, 57%), median age 70 years (range 40–89 years). A majority had pathologically confirmed non-squamous NSCLC (n = 143, 68%). Most patients had either pathological stage IIB (n = 81, 39%) or IIIA (n = 96, 46%) disease. The median days pre-operatively for [18F]FDG PET/CT being performed were 49 days (range 1–93 days).
A majority of patients had negative (TPS < 1%) PD-L1 expression determined by immunohistochemistry in the primary tumour (n = 111, 53%). Seventy-four (35%) had low (TPS 1–49%) PD-L1 expression and 25 (12%) had high (TPS ≥ 50%) PD-L1 expression. There was no statistical difference in clinical characteristics including age, sex, histopathological, tumour location or stage between the groups (Table 1). The only significant difference between the groups was the nodal metastasis PD-L1 TPS (p < 0.001). This was also the case when comparing clinical characteristics by the nodal metastasis PD-L1 TPS group, with the only significant difference being primary tumour PD-L1 TPS (p < 0.001; Supplementary Table 1). This was related to the inter-lesional heterogeneity between primary tumour and nodal metastases within individuals. The frequency of heterogeneity based on primary tumour PD-L1 TPS groups of < 1%, 1–49% and ≥ 50% was 7% (n = 8 of 111), 41% (n = 30 of 74) and 56% (n = 14 of 25), respectively.
Metabolic parameters and PD-L1 expression by TPS
Correlations between TPS and measured metabolic parameters of primary tumour or nodal metastasis are summarised in Table 2. There was a positive correlation between TPS and primary tumour SUVmax (Spearman’s rho (r) = 0.20; p < 0.05), SUVmean (r = 0.20; p < 0.05), SUVpeak (r = 0.16; p < 0.05) and SULpeak (r = 0.15; p < 0.05). Similarly, there was a positive correlation between TPS and nodal metastasis SUVmax (r = 0.30; p < 0.05) and SUVmean (r = 0.35; p < 0.05). However, there was no correlation for SUVpeak nor SULpeak. There was no correlation between TPS and metabolic parameters of tumour burden and heterogeneity (MTV, TLG, HISUV) of the primary tumour nor nodal metastasis.
Metabolic parameters and PD-L1 expression by TPS categories
The mean metabolic parameter scores with their standard error for both primary tumour and nodal metastases according to all TPS categories are summarised in Table 3. The mean SUVmax of primary tumour (n = 210) increased according to the < 1%, 1–49% and ≥ 50% TPS groups, at 11.75, 13.66 and 15.19, respectively (p = 0.02). On multiple comparison testing, significance held between TPS groups < 1% and ≥ 50%, but not between 1–49% group with either the < 1% or ≥ 50% group (Fig. 3). There was a similar trend but with lower mean scores for involved lymph nodes (n = 91) with mean SUVmax of 6.21, 7.26 and 11.50 for lymph node TPS groups of < 1%, 1–49% and ≥ 50%, respectively (p = 0.03), but with no significant difference between individual TPS groups (Fig. 3).
Violin plots displaying primary lung tumour (a–d) and malignant lymph node (e–h) SUV-based metabolic parameter median and lower/upper quartiles (dashed lines) of PD-L1 TPS groups of < 1%, 1–49% and ≥ 50%. Metabolic parameters of (a) primary SUVmax (p < 0.05, H test = 7.60), (b) primary SUVmean (p < 0.05, H test = 7.72), (c) primary SUVpeak (p = 0.13, H test = 4.10), (d) primary SULpeak (p = 0.15, H test = 3.85), (e) lymph node SUVmax (p < 0.05, H test = 7.24), (f) lymph node SUVmean (p < 0.05, H test = 9.85), (g) lymph node SUVpeak (p < 0.05, H test = 11.17) and (h) lymph node SULpeak (p < 0.05, H test = 9.74). Lines above the plots demonstrate Dunn’s multiple comparison tests between two individual TPS groups, where ns is non-significant (p > 0.05) and * represents the level of significance (* is p < 0.05; ** is p < 0.01 and *** is p < 0.001). Individual graphs created in GraphPad Prism, and figure created with BioRender.com
The SUVmean of the primary tumour also increased according to TPS groups, with a mean SUVmean of 7.16, 8.37 and 9.23, respectively (p = 0.02). Multiple comparison testing demonstrated significant difference (p < 0.05) between the < 1% and ≥ 50% primary tumour TPS groups only. A similar significant trend was seen with mean SUVmean values of 3.82, 4.84 and 7.48 for nodal metastases according to TPS groups < 1%, 1–49% and ≥ 50%, respectively (p = 0.007). There was no significant difference in SUVmean scores between nodal TPS groups of 1–49% and ≥ 50%.
Although there was a trend toward increased mean SUVpeak of the primary lung tumour with increasing TPS group, this was not significant (p = 0.13). However, the trend of increased mean SUVpeak of the nodal metastasis with increasing lymph node TPS category was significant (p = 0.004). When adjusting SUVpeak for lean body mass, the mean SULpeak values for the primary lung tumour did not significantly differ between TPS groups (p = 0.15). The trend of increased mean SULpeak with increasing TPS group in nodal metastases was significant (p = 0.008).
There were no statistically significant differences between all TPS groups of the primary lung tumour and nodal metastases for mean MTV (p = 0.80; p = 0.71, respectively), TLG (p = 0.82; p = 0.47) and HISUV (p = 0.30; p = 0.97) (Supplementary Figure 1).
Metabolic parameters and PD-L1 expression by PD-L1 TPS of < or ≥ 1%
The clinical characteristics of patients using either primary lung tumour or nodal metastases by TPS groups < or ≥ 1% are presented in Supplementary Tables 2 and 3. The only characteristic of significance between TPS groups is the nodal metastasis and primary tumour TPS groups, respectively. The mean metabolic parameter scores with their standard error for both primary tumour and nodal metastases according to PD-L1 TPS categories of < or ≥ 1% are summarised in Table 4.
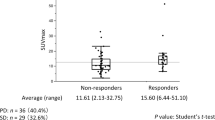
The SUVmax of primary tumour (n = 210) increased according to the TPS group < or ≥ 1%, with mean SUVmax of 11.75 and 14.05, respectively (p = 0.01; Fig. 4). Mean SUVmax measurements were also significantly higher in nodal metastases with PD-L1 ≥ 1% (8.29) compared to those with no PD-L1 expression < 1% (6.21) (p = 0.01). The SUVmean of the primary tumour was also higher in those with a TPS ≥ 1% at 8.59 compared to those with TPS < 1% at 7.16 (p = 0.009). Similarly, the mean SUVmean measurements were higher in the TPS ≥ 1% nodal metastasis group at 5.50 compared to the TPS < 1% group at 3.82 (p = 0.002).
Violin plots displaying primary lung tumour (a–d) and malignant lymph node (e–h) metabolic parameter median and lower/upper quartiles (dashed lines) of PD-L1 TPS groups of < 1% and ≥ 1%. Metabolic parameters of (a) primary SUVmax (p < 0.05), (b) primary SUVmean (p < 0.01), (c) primary SUVpeak (p < 0.05), (d) primary SULpeak (p = 0.05), (e) lymph node SUVmax (p < 0.05), (f) lymph node SUVmean (p < 0.05), (g) lymph node SUVpeak (p < 0.05) and (h) lymph node SULpeak (p < 0.05). Lines above the plots demonstrate the Mann-Whitney U test p values between the two TPS groups, where ns is non-significant (p > 0.05) and * represents the level of significance (* is p < 0.05; ** is p < 0.01 and *** is p < 0.001). Individual graphs created in GraphPad Prism, and figure created with BioRender.com
The mean SUVpeak of primary lung tumour with a TPS ≥ 1% was higher at 12.38 compared to those with a TPS < 1% at 10.43 (p = 0.04). A statistical difference of SUVpeak measurements was also present for nodal metastases in those with TPS ≥ 1% at 9.80 compared to those with TPS < 1% at 6.21 (p = 0.01). Adjusted for lean body mass, mean SULpeak measurements were also higher in primary tumours with positive TPS ≥ 1% at 8.98 compared to those with TPS < 1% at 7.67 (p = 0.05). Similarly, there was a significant difference in mean SULpeak measurements for nodal metastases with TPS ≥ 1% at 7.34 compared to those with TPS < 1% at 4.53 (p = 0.02).
There was no statistically significant difference between primary tumour TPS < 1% and ≥ 1% for the mean MTV (p = 0.88), TLG (p = 0.54) and HISUV (p = 0.12). There was also no difference between the nodal metastasis TPS groups < or ≥ 1% for mean MTV (p = 0.81), TLG (p = 0.34) and HISUV (p = 0.83) (Table 4; Supplementary Figure 2).
To determine the sensitivity and specificity of the metabolic parameters associated with TPS immunohistochemistry positivity at the 1% threshold, ROC and area under the curve (AUC) analyses were performed (Fig. 5). The AUCs for metabolic parameters SUVmax, SUVmean, SUVpeak and SULpeak for primary tumour were in range 0.58–0.60, and for nodal metastases in range 0.66–0.73. The AUC for primary tumour SUVmax at the 1% positive threshold was 0.60, with SUVmax > 9.13 providing a sensitivity of 77% and specificity of 41%. Similarly, the AUC for nodal SUVmax at the 1% threshold was 0.66, with SUVmax > 5.29 providing a sensitivity of 68% and specificity of 60%. Multivariate analysis was also performed for PD-L1 expression at the 1% threshold, with no factors of interest, including SUVmax and SUVmean, determined as independent predictors for the primary tumour. However, both age and SUVmean were demonstrated as significant independent predictors for malignant lymph node PD-L1 expression < or ≥ 1% (Supplementary Table 6).
ROC curves displaying both primary tumour (a–d) and malignant lymph node (e–h) significant metabolic parameters for PD-L1 TPS of < or ≥ 1%. Primary tumour (a) SUVmax (AUC 0.60; 95% CI 0.53–0.68; p = 0.01; cut-off value 9.13 with sensitivity 77% and specificity 41%), (b) SUVmean (AUC 0.60; 95% CI 0.53–0.68; p = 0.009; cut-off value 5.63 with sensitivity 78% and specificity 45%), (c) SUVpeak (AUC 0.58; 95% CI 0.50–0.66; p = 0.04; cut-off value 10.07 with sensitivity 61% and specificity 55%) and (d) SULpeak (AUC 0.58; 95% CI 0.50–0.66; p = 0.05; cut-off value 6.17 with sensitivity 73% and specificity 47%). Lymph node metastasis (e) SUVmax (AUC 0.66; 95% CI 0.54–0.77; p = 0.01; cut-off value 5.29 with sensitivity 68% and specificity 60%), (f) SUVmean (AUC 0.69; 95% CI 0.58–0.80; p = 0.003; cut-off value 3.84 with sensitivity 60% and specificity 71%), (g) SUVpeak (AUC 0.73; 95% CI 0.58–0.89; p = 0.01; cut-off value 5.50 with sensitivity 76% and specificity 65%) and (h) SULpeak (AUC 0.73; 95% CI 0.57–0.88; p = 0.02; cut-off value 3.79 with sensitivity 81% and specificity 57%). Individual graphs created in GraphPad Prism, and figure created with BioRender.com
Metabolic parameters and PD-L1 expression by PD-L1 TPS of < or ≥ 50%
The clinical characteristics of patients using either primary lung tumour or nodal metastases by TPS groups less than or greater than/equal to 50% are presented in Supplementary Tables 4 and 5. The mean metabolic parameter scores with their standard error for both primary tumour and lymph node metastases according to TPS categories of < or ≥ 50% are summarised in Supplementary Table 7. The results were more varied according to the 50% positivity threshold; e.g. the mean SUVmax was statistically higher in those with primary tumour TPS of ≥ 50% but not in nodal metastases (Supplementary Figures 3 and 4).
Discussion
This is the first study, to our knowledge, that demonstrates the association of semi-quantitative [18F]FDG-PET/CT metabolic parameters, namely SUVmax, SUVmean, SUVpeak and SULpeak, with PD-L1 expression in both primary tumour and nodal metastases in resectable NSCLC. Mean values of SUV-based metabolic parameters, including SUVmax, SUVmean, SUVpeak and SULpeak, all increased by TPS groups, but these differences were not significant on correcting for multiple comparisons. Using a PD-L1 positivity threshold of 1%, which is standard for the 22C3 assay used, all of these metabolic parameters were significantly higher in the TPS ≥ 1% group. Whilst it is possible that these parameters could be used to predict TPS groups as measured by immunohistochemistry, the sensitivity and specificity of these measurements were poor, for both primary tumour and nodal metastases. Using primary tumour SUVmean > 5.63 as a cut-off for TPS positivity ≥ 1%, the sensitivity was moderate at 78% but specificity was low at 45%, reflecting multifactorial causes for [18F]FDG uptake, beyond simply PD-L1 co-expression. Malignant lesion [18F]FDG SUV-based parameters alone are therefore unlikely to be sufficient in predicting PD-L1 positivity and, as such, anti-PD-1/PD-L1 therapeutic response. However, they have the potential to play an additive role, for example as part of a nomogram taking into account several other multi-modal predictive variables. Several studies have reported the potential utility of SUV-based parameters in predicting PD-L1 expression in the primary tumour and/or anti-PD-1/PD-L1 therapy response, primarily in advanced disease [16,17,18,19,20,21,22,23,24]. A large study (n = 374) of non-selected all-stage NSCLC reported SUVmax > 12.5 was predictive of PD-L1 expression, with a sensitivity of 65.4% and specificity of 86.7% [23]. Across the literature, SUV-based parameters, particularly SUVmax, appear to be most consistently associated with PD-L1, despite the heterogeneity of patient and histological characteristics within and between studies. This is unsurprising given that several preclinical studies have demonstrated that PD-L1 itself promotes expression of GLUT1 and HK2, and thus glycolysis [14, 30].
There are a small number of studies reporting an association of [18F]FDG-PET/CT parameters in early-stage lung cancer with PD-L1, but only in the primary tumour. For example, Kaira et al demonstrated the association of high PD-L1 with [18F]FDG uptake using SUVmax in 315 patients with lung adenocarcinoma [31]. However, the study appeared to use multiple assays and a non-clinically validated scoring mechanism, defining PD-L1 high as membranous staining ≥ 6%. Another study of 548 patients with resected NSCLC demonstrated that pre-operative SUVmax was significantly higher in those with a PD-L1 expression ≥ 5% using the SP142 assay [32]. Whilst several studies have shown comparability of most PD-L1 assays (22C3, 28-8, SP263), the SP142 assay does not appear to be comparable, with fewer stained tumour cells [33]. Additionally, the approved cut-off for positivity of PD-L1 expression in NSCLC is either ≥ 50% tumour cells or ≥ 10% immune cells. With a lower, ≥ 5%, cut-off used in this study, it is unclear whether the association of higher SUVmax with PD-L1 positivity described has clinical utility.
[18F]FDG-PET/CT measures of tumour burden have generally been investigated in terms of immune checkpoint inhibitor response than with PD-L1 expression. Similar to our findings, a study of 32 patients with advanced NSCLC described higher whole-body MTV and TLG in those with positive PD-L1 expression, but not statistically different in the small sample [19]. This is unsurprising as nodal staging in particular focuses on number and location of involved lymph nodes, rather than size, so it is possible to get a wide spectrum of nodal metabolic volumes, i.e. tumour burden, even in early-stage disease. Smaller disease volumes, associated with early-stage disease, are also more prone to partial volume effects and an underestimation of activity concentrations in the analysed image reconstructions [34].
The 1% threshold for PD-L1 positivity in NSCLC is of particular importance considering that several clinical trials have demonstrated that it is associated with clinical benefit to anti-PD-1/PD-L1 therapies in the perioperative, neoadjuvant and adjuvant settings [5, 10, 28]. Our study was conducted in a retrospective cohort preceding routine anti-PD-1/PD-L1 therapy use in early-stage NSCLC, and as such, it was not possible to determine whether these metabolic parameters could predict response and survival associated with anti-PD-1/PD-L1 treatment. This has, however, been demonstrated in advanced NSCLC in several studies [17,18,19, 35,36,37,38,39]. For example, Takada et al, in 89 patients with advanced NSCLC receiving anti-PD-1 therapy, found that pre-treatment SUVmax ≥ 11.16 was significantly associated with higher response rate (41.3%) compared to those with an SUVmax < 11.16 (11.6%) [17]. However, the studies are of small samples and there are conflicting results which add additional complexity to understanding the prognostic role these imaging biomarkers could play, in what is an increasingly diverse multi-modality treatment landscape.
Alternative approaches, such as radiomics or deep learning, provide further opportunity. For example, a heterogenous (all-stage) study of 334 patients with NSCLC demonstrated improved prediction of PD-L1 expression using a radiomics model derived from two optimal features extracted from [18F]FDG PET and CT components [40]. It may also be of interest to combine those [18F]FDG SUV-based parameters associated with PD-L1 expression with other markers of the immune microenvironment, for prediction of anti-PD-1/PD-L1 therapy response. These could include, within a predictive nomogram, SUV-based bone marrow-to-spleen ratio, a surrogate marker of haematopoiesis and immune function, histopathological assessment of tumour infiltrating lymphocytes, tumour mutation burden, a genomic marker of immunogenicity and even clinical factors, such as, smoking status [41,42,43].
Our study has some limitations. Firstly, this is a single-centre retrospective study, mitigated by the fact that our tertiary centre covers referrals from a wide geographical location across South-East England. Secondly, the SUV was derived from a fixed threshold volume of interest and is therefore potentially affected by the partial volume effect in smaller lesions. Despite the large cohort, the number of PET measurable lymph nodes was limited (n = 91) and, as such, it would be important to validate the study’s findings in a larger cohort; this is especially important as these small lesions are more adversely affected by partial volume effect [34]. [18F]FDG-PET/CT was performed up to 3 months prior to surgery in this study; there is potential for differences in tumour microenvironment PD-L1 expression at the time of imaging and at tissue retrieval. Of note, we only investigated the association of [18F]FDG-PET/CT metabolic parameters with PD-L1 expression as TPS, i.e. tumour cell expression, and using one assay. The SUV measurements of primary tumour and nodal metastases are in fact indicative of [18F]FDG uptake within the wider tumour microenvironment, which includes other immune cells, such as tumour infiltrating lymphocytes, that use glycolytic metabolism. As such, it would be useful for further studies to investigate the association of said metabolic parameters with total PD-L1 expression (i.e. tumour and immune cells) and across PD-L1 assays.
Conclusions
With the ever-increasing role of immune checkpoint inhibitors in the perioperative setting of NSCLC, it is important to understand the heterogeneity and role of PD-L1 expression in early-stage disease, and [18F]FDG-PET/CT may play an important role. This study demonstrated an association of standard [18F]FDG-PET/CT metabolic parameters with PD-L1 expression in both primary tumour and lymph node metastasis of resectable NSCLC. However, the sensitivity and specificity of these measurements for predicting PD-L1 positivity using the 1% threshold were poor. Future prospective studies are warranted to definitively determine the association, predictive role and, as such, clinical utility of these [18F]FDG-PET/CT-based biomarkers for PD-L1 expression and/or PD-L1-directed therapy. Due to the complexity of the tumour microenvironment, it is unlikely that non-invasive [18F]FDG-PET/CT metabolic parameters will replace immunohistochemical techniques, or other direct PD-L1 measurement methods alone, but they may supplement them, for example as part of a predictive marker nomogram alongside patient and biological characteristics.
Abbreviations
- [18F]FDG:
-
Fluorine 18 fluorodeoxyglucose
- CT:
-
Computed tomography
- GLUT1:
-
Glucose transporter 1
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- HISUV:
-
SUV-based heterogeneity index
- HK2:
-
Hexokinase II
- MTV:
-
Metabolic tumour volume
- NSCLC (NOS):
-
Non-small cell lung cancer (not otherwise specified)
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death-ligand 1
- PET:
-
Positron emission tomography
- SUL:
-
SUV adjusted for lean body mass
- SUV:
-
Standardised uptake value
- TLG:
-
Total lesion glycolysis
- TPS:
-
Tumour proportion score
- VOI:
-
Volume of interest
References
Reck M, Rodríguez-Abreu D, Robinson AG et al (2019) Updated analysis of KEYNOTE-024: pembrolizumab versus platinum based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37(7):537–546
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265
Vokes EE, Ready N, Felip E et al (2018) Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29(4):959–965
Fehrenbacher L, Spira A, Ballinger M et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open label, phase 2 randomised controlled trial. Lancet 387(10030):1837–1846
Spigel DR, Faivre-Finn C, Gray JE et al (2022) Five-year survival outcomes from the PACIFIC Trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol 40(12):1301–1311. Erratum in: J Clin Oncol 40(17):1965
de Castro G, JKudaba I Jr, Wu YL et al (2023) Five-year outcomes with pembrolizumab versus chemotherapy as first line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 study. J Clin Oncol 41(11):1986–1991
Herbst RS, Baas P, Kim D-W et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
McLaughlin J, Han G, Schalper KA et al (2016) Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol 2(1):46–54
Hughes DJ, Subesinghe M, Taylor B et al (2022) 18F FDG PET/CT and novel molecular imaging for directing immunotherapy in cancer. Radiology 304(2):246–264
Forde PM, Spicer J, Lu S et al (2022) Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 386(21):1973–1985
Pak K, Park S, Cheon GJ et al (2015) Update on nodal staging in non-small cell lung cancer with integrated positron emission tomography/computed tomography: a meta-analysis. Ann Nucl Med 29:409–419
Salas JR, Clark PM (2022) Signaling pathways that drive 18F-FDG accumulation in cancer. J Nucl Med 63(5):659–663
DeBerardinis RJ, Chandel NS (2020) We need to talk about the Warburg effect. Nat Metab 2(2):127–129
Li J, Chen R, Chen Y et al (2023) Relationship between the expression of PD-L1 and 18F-FDG uptake in pancreatic ductal adenocarcinoma. Br J Cancer 129:541–550
Chang YL, Yang CY, Lin MW, Wu CT, Yang PC (2016) High co-expression of PDL1 and HIF-1a correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 60:125–135
Takada K, Toyokawa G, Okamoto T et al (2017) Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med 6(11):2552–2561
Takada K, Toyokawa G, Yoneshima Y et al (2019) 18F-FDG uptake in PET/CT is a potential predictive biomarker of response to anti-PD-1 antibody therapy in non-small cell lung cancer. Sci Rep 9(1):13362
Hashimoto K, Kaira K, Yamaguchi O et al (2020) Potential of FDG-PET as prognostic significance after anti-PD-1 antibody against patients with previously treated non-small cell lung cancer. J Clin Med 9(3):725
Evangelista L, Cuppari L, Menis J et al (2019) 18F-FDG PET/CT in non-small-cell lung cancer patients: a potential predictive biomarker of response to immunotherapy. Nucl Med Commun 40(8):802–807
Monaco L, Gemelli M, Gotuzzo I et al (2021) Metabolic parameters as biomarkers of response to immunotherapy and prognosis in non-small cell lung cancer (NSCLC): a real world experience. Cancers (Basel) 13(7):1634
Evangelista L (2019) The prediction of response to immunotherapy in nonsmall cell lung cancer patients by 18F-FDG PET/CT. J Thorac Dis 11(11):E221–E223
Jreige M, Letovanec I, Chaba K et al (2019) 18F-FDG PET metabolic-to-morphological volume ratio predicts PD-L1 tumour expression and response to PD-1 blockade in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 46(9):1859–1868
Wu X, Huang Y, Zhao Q et al (2020) PD-L1 expression correlation with metabolic parameters of FDG PET/CT and clinicopathological characteristics in non-small cell lung cancer. EJNMMI Res 10(1):51
Kaira K, Kuji I, Kagamu H (2021) Value of 18F-FDG-PET to predict PD-L1 expression and outcomes of PD-1 inhibition therapy in human cancers. Cancer Imaging 21(1):11
Moss C, Haire A, Cahill F et al (2020) Guy’s cancer cohort – real world evidence for cancer pathways. BMC Cancer 20:187
Agilent Technologies, PD-L1 IHC 22C3 pharmDx interpretation manual – NSCLC, Agilent Technologies (2021) Available via https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf. Accessed 14 Sep 2023
Provencio M, Nadal E, González-Larriba JL et al (2023) Perioperative nivolumab and chemotherapy in stage III non-small cell lung cancer. N Engl J Med 389:504–513
Felip E, Altorki N, Zhou C et al (2021) Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398(10308):1344–1357
Boellaard R, Delgado-Bolton R, Oyen WJ et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42(2):328–354
Chang CH, Qiu J, O’Sullivan D et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241
Kaira K, Shimizu K, Kitahara S et al (2018) 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur J Cancer 101:181–190
Takada K, Toyokawa G, Tagawa T et al (2017) Association between PD-L1 expression and metabolic activity on 18F-FDG PET/CT in patients with small-sized lung cancer. Anticancer Res 37(12):7073–7082
Hirsch FR, McElhinny A, Stanforth D et al (2017) PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12(2):208–222
Soret M, Bacharach SL, Buvat I (2007) Partial-volume effect in PET tumour imaging. J Nucl Med 48(6):932–945
Grizzi F, Castello A, Lopci E (2018) Is it time to change our vision of tumor metabolism prior to immunotherapy? Eur J Nucl Med Mol Imaging 45(6):1072–1075
Ke L, Wu L, Yu J, Meng X (2021) Feasibility of semiquantitative 18F-fluorodeoxyglucose PET/computed tomography in patients with advanced lung cancer for interim treatment evaluation of combining immunotherapy and chemotherapy. Nucl Med Commun 42(9):1017–1023
Kaira K, Higuchi T, Naruse I et al (2018) Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging 45(1):56–66
Polverari G, Ceci F, Bertaglia V et al (2020) (18)F-FDG Pet parameters and radiomics features analysis in advanced NSCLC treated with immunotherapy as predictors of therapy response and survival. Cancers (Basel) 12(5)
Ke L, Wang L, Yu J, Meng X (2021) Prognostic significance of SUVmax combined with lactate dehydrogenase in advanced lung cancer patients treated with immune checkpoint inhibitor plus chemotherapy: a retrospective study. Front Oncol 11:652312
Zhao X, Zhao Y, Zhang J, Zhang Z, Liu L, Zhao X (2023) Predicting PD-L1 expression status in patient with non-small cell lung cancer using [18F]FDG PET/CT radiomics. EJNMMI Res 13(1):4
Seban RD, Assié JB, Giroux-Leprieur E et al (2021) Prognostic value of inflammatory response biomarkers using peripheral blood and [18F]-FDG PET/CT in advanced NSCLC patients treated with first-line chemo- or immunotherapy. Lung Cancer 159:45–55
Yi M, Jiao D, Xu H et al (2018) Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 17:129
Xiao J (2020) Combination of clinical factors and PET/CT features can predict positive PD-L1 for patients with lung adenocarcinoma: a novel approach based on 18F-FDG PET/CT nomogram. J Nucl Med 61(supplement 1):1332
Acknowledgements
The authors would like to thank our patients and colleagues at King’s Health Partners Comprehensive Cancer Centre and the Cancer Centre at Guy’s, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
Funding
The authors acknowledge financial support from Merck, Sharp & Dohme (MSD) with a research grant specifically for this study via institution, the Cancer Research UK National Cancer Imaging Translational Accelerator (C1519/A28682), and the Wellcome/Engineering and Physical Sciences Research Council Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z). For the purpose of open access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
DJH, EJ, RO, TM, CH, SH, PT, DN, VG, AB, EK and GJRC were involved in data curation, formal analysis, investigation and methodology; DJH, EK and GJRC were involved in conceptualisation and writing – reviewing and editing; DJH and EJ were involved in project administration; DJH was involved in validation, visualisation and writing – original draft; EK and GJRC were involved in supervision. All authors reviewed the final manuscript and agreed to publication; DJH, EK and GJRC made the final decision to submit.
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Gary Cook (GJRC).
Conflict of interest
DJH has received honoraria/speaker fees from Pfizer and Bristol Myers Squibb, transport/accommodation grants from Bristol Myers Squibb, Roche and NanoMab Technology and research funding via institute from NanoMab Technology, and is an executive committee member of the Association of Cancer Physicians (UK). EK has received research funding via institute from Merck, Sharp & Dohme (MSD). GJRC has received research support from NanoMab Technology, Theragnostics and Serac Healthcare, and provides consultancy for GE Healthcare, NanoMab Technology, Amgen, Blue Earth Diagnostics and Full-Life Technologies. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study as per the institutional review board.
Ethics approval
The study underwent ethics review and was approved by UK Research Ethics Committee (UK IRAS 228790) and within the previously described Guy’s Cancer Cohort framework (ref: 18/NW/0297).
Study subjects or cohort overlap
No study subjects have been previously reported to the best of the authors’ knowledge.
Methodology
• This is a retrospective observational study performed at one institution involving individual subjects from several referring centres.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eleni Karapanagiotou, Gary J. R. Cook are joint senior authors and both senior authors contributed equally.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hughes, D.J., Josephides, E., O’Shea, R. et al. Predicting programmed death-ligand 1 (PD-L1) expression with fluorine-18 fluorodeoxyglucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) metabolic parameters in resectable non-small cell lung cancer. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10651-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10651-5