Abstract
Objectives
The incidence of anal squamous cell carcinoma (ASCC) is increasing worldwide, with a significant proportion of patients treated with curative intent having recurrence. The ability to accurately predict progression-free survival (PFS) and overall survival (OS) would allow for development of personalised treatment strategies. The aim of the study was to train and external test radiomic/clinical feature derived time-to-event prediction models.
Methods
Consecutive patients with ASCC treated with curative intent at two large tertiary referral centres with baseline FDG PET-CT were included. Radiomic feature extraction was performed using LIFEx software on the pre-treatment PET-CT. Two distinct predictive models for PFS and OS were trained and tuned at each of the centres, with the best performing models externally tested on the other centres’ patient cohort.
Results
A total of 187 patients were included from centre 1 (mean age 61.6 ± 11.5 years, median follow up 30 months, PFS events = 57/187, OS events = 46/187) and 257 patients were included from centre 2 (mean age 62.6 ± 12.3 years, median follow up 35 months, PFS events = 70/257, OS events = 54/257). The best performing model for PFS and OS was achieved using a Cox regression model based on age and metabolic tumour volume (MTV) with a training c-index of 0.7 and an external testing c-index of 0.7 (standard error = 0.4).
Conclusions
A combination of patient age and MTV has been demonstrated using external validation to have the potential to predict OS and PFS in ASCC patients.
Clinical relevance statement
A Cox regression model using patients’ age and metabolic tumour volume showed good predictive potential for progression-free survival in external testing. The benefits of a previous radiomics model published by our group could not be confirmed on external testing.
Key Points
• A predictive model based on patient age and metabolic tumour volume showed potential to predict overall survival and progression-free survival and was validated on an external test cohort.
• The methodology used to create a predictive model from age and metabolic tumour volume was repeatable using external cohort data.
• The predictive ability of positron emission tomography-computed tomography–derived radiomic features diminished when the influence of metabolic tumour volume was accounted for.
Similar content being viewed by others
Introduction
Anal carcinoma, although rare, is increasing in incidence worldwide with the most common histological type being squamous cell carcinoma (ASCC) [1,2,3]. Since three landmark randomised trials between 1987 and 1994, the gold standard treatment for non-metastatic ASCC is chemoradiotherapy with only very early anal margin tumours being excised [4,5,6,7]. Following the adoption of chemoradiotherapy as the standard treatment, the rate of loco-regional failure (LRF) decreased and there was improvements in overall survival (OS) and cancer-specific survival. However, there are still approximately 16% of patients with LRF, and 5-year mortality remains 25% [8]. The ability to accurately predict outcome at diagnosis could guide more tailored treatment, help stratify surveillance plans and ultimately improve outcomes.
European Society for Medical Oncology (ESMO) guidelines recommend pre-treatment use of multi-parametric magnetic resonance imaging (MRI) and 2-deoxy-2-[fluorine-18]fluoro-d-glucose (FDG) positron emission tomography/computed tomography (PET-CT) to assess regional and distant disease [9, 10]. These images provide the opportunity to extract quantitative data which can be used as features within a predictive model, a process termed as radiomics [11]. Although use of radiomics is widely reported for assessment and prediction in many different disease processes, limited data is available on outcome prediction models in ASCC using radiomic features extracted from PET-CT [12]. Brown et al used an elastic net model, combining least absolute shrinkage and selection operator (LASSO) and ridge regression for selection of radiomic features to predict progression-free survival (PFS) in ASCC patients treated with radiotherapy, mitomycin C and 5-fluorouracil regimens [13]. The study was based on retrospective data from a large tertiary centre with 145 patients in the training dataset and 44 patients in the internal test set. The model achieved a training area under the curve (AUC), based on the receiver operator characteristic (ROC) curve, of 0.74 and a test AUC of 0.73. However, the model was not externally validated. This is often a limitation in the published literature and means that it is not possible to determine how generalisable or universally applicable the reported model is [14].
This study aimed to derive and externally validate a predictive model for OS and PFS in ASCC patients using data from two large tertiary centres within the UK, Leeds Teaching Hospitals NHS Trust (LTHT) and the Christie NHS Foundation Trust (CNFT).
Material and methods
The transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines were adhered to as part of the study (Supplementary Material 1).
Patient selection
Consecutive patients with histological proven ASCC who underwent pre-treatment FDG PET/CT at LTHT between June 2008 and January 2017, or at CNFT between January 2012 and January 2018, were included. This allowed sufficient follow-up time for events to present in this cohort of patients. Exclusion criteria included patients with no definable tracer uptake in the primary tumour; treatment prior to PET-CT; patients not treated with curative intent; patients with incomplete clinical datasets; or if the primary lesion was too small to accurately segment on imaging.
Patient age, gender, clinical history, treatment history, clinical outcome and follow-up duration were recorded from the electronic patient records. PFS was recorded as any relapse, recurrence, or death from any cause. OS was recorded as death from any cause.
Ethical consideration
All patients included within the study gave prospective consent at the time of imaging for their anonymised FDG PET-CT imaging data to be used in research and service development projects. Formal ethics committee approval was waived for this study for LTHT patients, as it was considered by the institutional review board to represent evaluation of a routine clinical service. For CNFT patients, use of data was approved by the institutional UK Computer Aided Theragnostics (ukCAT) ethics committee.
Radiomic feature analysis
The details of the imaging protocols and who performed the segmentation and interpretation of images is available in Supplementary Material 2.
Image segmentation and resampling
Lesions were segmented with a semi-automated process using Local Image Features Extraction software (LIFEx v4.0, www.lifexsoft.org) [15]. There is no consensus on the optimal segmentation methodology in this clinical scenario. Whilst a fixed threshold may be easily applied, there is growing evidence that thresholding adapted to background physiological uptake might be more patient specific. In previous work, we have employed this segmentation methodology and have generally found that a threshold adapted to background physiological liver tracer uptake gives a good representation of lesions without background involvement which correlates to anatomical volumes on the CT component [13, 16, 17] (Fig. 1). Primary tumour region of interests ROI (t-ROI) and separate lymph-node ROI (ln-ROI) were contoured using a threshold of 1.5 × mean liver standardised uptake value (SUV) [18]. PET contours were transposed onto the co-registered CT to create CT ROIs. No lesions were excluded from analysis using this chosen segmentation threshold in the study cohort.
For each PET ROI, the voxel value was resampled into 64 bins ranging from 0 to 20 SUVs. The CT voxel values were resampled into 400 bins ranging from − 1000 to 3000 Hounsfield units (HU). For the LTHT dataset, the voxel dimensions were resampled to 4.0 × 4.0 × 4.0 mm for PET images and 2.5 × 1.2 × 1.2 mm (4.0 × 1.2 × 1.2 mm before June 2014) for CT images. For the CNFT dataset, the voxel dimensions were resampled to 4.0 × 4.0 × 4.0 mm for PET and CT images. Uniform resampling has been demonstrated to improve robustness in radiomic features when images are acquired using different voxel sizes [19].
Feature extraction
Feature extraction was performed using LIFEx software which adheres to the image biomarker standardisation initiative (IBSI) [20]. Forty-four features were extracted for each PET and CT t-ROI, listed in Supplementary Material 2. Radiomic features were not extracted from the ln-ROI, but the volume of the ROI was used to calculate the total metabolic tumour volume (MTV).
Statistical analysis and predictive modelling
Each model was trained, and hyperparameters tuned where applicable, initially on a single site, either LTHT or CNFT. Modelling and statistical analysis were performed using both R v4.0 and Python v3.7. A significant p value was taken as 0.05 except when Bonferroni correction was applied. Hazard ratios (HR) with 95% confidence intervals and c-indices with standard error were reported.
CNFT predictive models
Firstly, a multivariable Cox proportional hazards (PH) regression model was created using clinical variables, tumour (T) stage, lymph node (N) stage, age and MTV using only complete cases. T and N-stage, age and MTV were considered continuous variables. The proportional hazards assumption was assessed graphically, and variables transformed if necessary. Secondly, unsupervised, and supervised learning methods were then used to explore the development of a radiomics-based Cox model. The unsupervised approach used principal component analysis (PCA) on the correlation matrix to reduce the dimensionality of the radiomics dataset. The leading principal components that accounted for 80% of the variability were explored using a Cox PH model with/without adjusting for MTV for both PFS and OS. The supervised learning approach involved exploring each radiomic feature in univariable analysis with/without adjusting for MTV. Any significant features were adjusted for the baseline clinical model for OS or PFS. For each final model, calibration plots comparing observed versus fitted survival probabilities over time were assessed.
LTHT predictive models
A more automated pipeline with integrated harmonisation of the data for training and tuning of ML models for prediction of OS and PFS was explored. Models were created by dummy encoding the categorical clinical features (pandas.get_dummies v1.2.1), scaling the continuous features (sklearn.preprocessing.standardscaler v0.24.1) and harmonising the PET-CT radiomic features by applying neuroCombat to the extracted data (https://github.com/Jfortin1/ComBatHarmonization). To avoid redundancy, features with a Pearson coefficient of > 0.8 were removed. Three different ML models were evaluated on the dataset: survival support vector machine (SSVM), survival random forest (SRF) and Cox regression (scikit-survival v0.14.0).
A forward wrapper method was implemented for feature selection using threefold cross-validation (sklearn.model_selection.repeatedkfold v0.24.1). The optimum number of features for the greatest c-index for each ML algorithm was selected and hyperparameters were tuned using a grid search with a threefold cross-validation with 10 repeats (sklearn.model_selection.gridsearchcv v0.24.1).
External testing
No data extracted from the images were shared between sites. The models with the highest c-index for each method created at a single institution were re-tested on external data at the other institution. Kaplan–Meier survival plots were produced from the external test-set.
Repeatability of the methodology
The methodology for the best overall performing prediction model from either site was applied to the dataset of the other institution to determine if the same features would be selected and if the model would achieve a similar prediction score when trained on a different study population.
Results
Patient demographics
One hundred and eighty-seven patients were included from LTHT and 257 patients included from CNFT, with the median follow-up time being 30 months (IQR = 38 months) at LTHT and 45 months (IQR = 27 months) at CNFT. The breakdown of patient demographics is included in Table 1.
CNFT predictive models
Baseline clinical model
All features within the clinical model were significant predictors of OS and PFS following univariable analysis (Table 2). M stage was not included in the univariable analysis due to the low prevalence within the dataset (8/257). Treatment regime was not explored as a feature in the model due to the insufficient variance to provide meaningful information to the models being trained. Following multivariable analysis, it was found that only MTV and age remained significant predictors of OS and PFS. Furthermore, MTV correlated strongly with T, N-stage and MRI size (maximum single axis dimension recorded following MDT review). Therefore, the final clinical model consisted of just age and MTV (Table 3), which achieved a c-index of 0.70 and 0.68 for OS and PFS on the training data respectively.
Unsupervised radiomics model
The first 5 principal components accounted for 82% of the variability. The correlation of the leading components is seen in Table 4 and shows that once adjusted for MTV, none of the leading components were associated with OS or PFS. Therefore, this model was not explored any further within the analysis.
Supervised radiomics model
Each individual radiomic variable was individually explored in univariable analysis before and after adjusting for MTV. These results are visualised in the volcano plot in Fig. 2. Allowing for multiple testing (Bonferroni adjusted p value of 0.05, green line on plots below) but before adjustment for MTV, many radiomic features significantly correlate with both OS and PFS (black circles). However, the majority no longer do so once adjusted for MTV (red circles). Of interest, selected lower order PET features, total lesion glycolysis (TLG), SUV standard deviation and SUVpeak were close to the Bonferroni threshold for OS (TLG) and PFS (SUV standard deviation and SUVpeak), respectively.
Upon adjustment for the clinical OS and PFS models, only TLG gave a modest improvement over the clinical model with no feature improving the PFS model. Therefore, a multivariable Cox regression model including age, MTV and TLG was created (Table 5) which achieved a c-index of 0.70 on the training dataset.
External testing
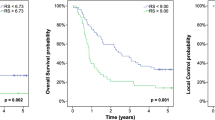
Both the clinical and combined clinical- and radiomic-based OS models achieved a c-index of 0.70 on the training data and therefore were both tested on the LTHT dataset, with the clinical based model achieving a slightly higher c-index of 0.70 (S.E. = 0.4) compared to 0.69 (S.E. = 0.4) for the combined model. For both these models, the predictions of the low- and high-risk groups follow the actual event rates; however, the medium-risk group was either overestimated, age and MTV model, or underestimated, age, MTV and TLG. Also, the confidence intervals overlapped between all groups for both models, meaning no distinct groups could be defined (Fig. 3).
Plot showing the observed overall survival probabilities over time (dotted lines) with 95% confidence intervals (shaded region) and the predicted survival probabilities (solid lines) for a model created using MTV and age. Green = low-risk group, red = medium-risk group and black = high-risk group. Figure derived from the results of the testing on the LTHT dataset
When predicting PFS using a model derived from MTV and age, the c-index was 0.70 (S.E. = 0.4) on the test data with the model greatly overestimating the number of events of the medium-risk group (Fig. 4).
Plot showing the observed PFS over time (dotted lines) with 95% confidence intervals (shaded region) and the predicted (solid lines) for a model created using MTV and age. Green = low-risk group, red = medium-risk group and black = high-risk group. Figure derived from the results of testing on the LTHT dataset
LTHT predictive models
When using a forward wrapper method and ComBat harmonisation to explore three ML methods on the LTHT data, it was found that the best predictive model for OS was created using a SRF model with the parameters CT-derived neighbourhood grey-level difference matric (NGLDM) coarseness, age, CT-derived grey-level run length matrix (GLRLM), high grey run emphasis (HGRE), PET-derived grey-level co-occurrence matrix (GLCM) entropy log10 and CT-derived GLRLM short-run high grey-level emphasis (SRHGE). The hyperparameters used were random state = 0, bootstrap = True, maximum depth = 2, minimum number of samples per split = 4, maximum features = 5, minimum samples per leaf = 2, number of estimators = 50 and out of bag score = False. This led to a mean training c-index of 0.74.
No combination of features, ML model or hyperparameter selection allowed for the creation of a PFS model with a c-index above 0.55 without demonstrating obvious signs of overfitting (mean training and validation scores having a difference of over 0.20) and therefore no PFS model was tested on the external dataset.
External testing
The radiomic-based SRF OS prediction model had a test c-index of 0.60 when applied to the CNFT dataset. No PFS model was tested on the external dataset due to overfitting on the training dataset.
Repeatability of the methodology
When applying the best performing CFNT methodology to train a model on the LTHT dataset, the same parameters MTV and age were selected from the univariable and multivariable analysis. The models produced a test c-index of 0.7 (S.E. = 0.04) for both PFS and OS when tested on the CNFT test set (Figs. 5 and 6). The predicted and observed survival probabilities were in good agreement for OS but not for PFS.
Plot showing the observed overall survival probabilities over time (dotted lines) with 95% confidence intervals (shaded region) and the predicted (solid lines) for a model created using MTV and age. Green = low-risk group, red = medium-risk group and black = high-risk group. Figure derived from the results of testing on the CNFT dataset
Plot showing the observed fraction progression free over time (dotted lines) with 95% confidence intervals (shaded region) and the predicted (solid lines) for a model created using MTV and age. Green = low-risk group, red = medium-risk group and black = high-risk group. Figure derived from the results of testing on the CNFT dataset
When investigating MTV and its relationship with outcomes, it was demonstrated that both cohorts had a non-linear relationship and therefore no definitive cutoff could be recommended. Representative plots are provided in Supplementary Material 3.
Discussion
This study highlights MTV and age as biomarkers for predicting outcome in patients with ASCC, with MTV having a 1.49 HR and age having a 1.05 HR on multivariate analysis for OS and 1.69 HR and 1.03 HR for PFS, respectively. When combined in a Cox regression model, they had a good predictive ability with a c-index of 0.7 for both OS and PFS. Our study also illustrates the limitations of more complex radiomic based models when applying them to different populations.
The potential use of MTV for outcome prediction in this setting was first reported by Bazan et al in a small dataset of 39 patients [21]. Subsequent studies have explored the use of PET-CT-derived parameters in predictive models; however, due to the relatively low incidence of ASCC, study cohort sizes and event rates have been small. Of note, Rusten et al found that MTV, TLG and the Z-normalised combination of MTV and SUVpeak were predictive of outcomes, but the strongest predictors were nodal stage 3 disease and human papilloma virus (HPV) status [22]. Unfortunately, due to missing data within our cohort, it was not possible to explore HPV status further. Jones et al, in a prospective study, also reported that the predictive ability of MTV derived using a 41% SUVmax fixed threshold had excellent predictive ability for recurrence with an AUC of 0.89; however, this study only included 19 patients having a follow-up PET-CT 12 weeks after treatment [23]. Conversely, a larger study by Braun et al assessing outcomes in 60 patients found that MTV when split into two groups around the mean correlated well with T stage; however, there was no significant difference between disease-free survival [24].
There are various semi-automated segmentation techniques reported in the literature based on using a fixed SUV threshold, an adaptive threshold related to lesions being contoured or related to background physiological uptake. As far as we are aware, there is no published work comparing different segmentation approaches in anal cancer. Recent work defining the optimal method for MTV assessment of cervical cancer from pre-treatment FDG PET-CT evaluated different fixed and gradient segmentation methods showing excellent inter-observer agreement across all thresholds [25].
Our prior study exploring PET-CT-derived predictive modelling using radiomics in ASCC patients used the same 189 patient cohort as further analysed here [13]. We found that a regression-based model created from a combination of 10 different clinical and radiomic parameters, including MTV, had an AUC of 0.74 for the training and validation cohorts when predicting PFS. However, in the current study, we were not able to replicate this performance level, likely due to the presence of overfitting in the initial study, which was not identified as the model was trained and tested once on an internal dataset with a relatively small number of events. The issue of overfitting is highlighted in the current study whereby creation of models using a forward wrapper feature selection method in combination with a ML algorithm mean training and validation c-indices were > 0.7; however, when tested on external data, the c-index dropped to 0.6 at best. This is likely a combination of the limited number of events and the use of a relatively large number of parameters. To improve the model, a larger dataset with more events is required, which may allow the number of features used to create the model to be limited and a greater number of repeats of the cross validation could be performed to give a better estimation of how the model is performing [26]. This also illustrates the need for an external test set, as highlighted within the radiomics quality score, when evaluating predictive models as it is not possible to determine how generalisable the models are without these even with the use of cross-validation to assess stability [27]. However, it is noted that there is a relatively small number of ML studies which have published external testing [28, 29].
An MTV and age-based predictive model represents a simpler model which may be more generalisable than a complex model incorporating radiomic features. This could be a more pragmatic route to clinical translation but requires further validation in a multi-centre study. This aligns well with work being undertaken by the Anal cancer Treatment Outcome Modelling with Computer Aided Theragnositics (AtomCAT) consortium who have developed the infrastructure to support testing and validation of a clinical prediction model in anal cancer [30]. This presents an opportunity to test the applicability of a combined clinical and MTV-based model across a wide range of treatment centres. If proven to be more universally applicable, the model could have a significant impact on patient outcomes by guiding risk-adapted therapy and more personalised follow-up.
In our study, it was found that the predictive power of radiomics was lost when correcting for MTV and that the variation of the radiomic features was largely explained by MTV. This relationship was concordant across both sites data. However, this does not mean radiomic analysis should be discounted from future studies. The study limited itself to 44 features extracted from both the PET and CT components and used a fixed bin number of 64 bins and 400 bins when creating the matrix derived parameters for PET and CT, respectively. Numerous additional radiomic features have not been explored. There is no consensus on the optimum bin number or bin width, especially in ASCC; therefore, it may be prudent in future studies to look at the robustness of radiomic features in this clinical setting when using different bin widths [31, 32]. Also, when performing the initial univariable and multivariable analysis to assess the relationship of the features with outcome, only monotonic relationships were explored. Although the use of feature selection with models such as kernel version of the SSVM and SRF should negate this, the relationships between features and outcomes could be explored further.
Interpretation of the clinical significance of a prediction model can be challenging, and net benefit analysis can be used to determine clinical value [33]. This incorporates disease prevalence and weighting for consequences of false positive or false negative results and may provide a more representative measure of the clinical utility of including or excluding a test. Plotting net benefit over a range of appropriate weights to derive a decision curve could then be performed. This aspect was beyond the scope of the current work and is a study limitation.
Another consideration is the choice of outcome metrics, as the specific time that an event is recorded/diagnosed can vary greatly from when the patient developed recurrence or relapsed [34]. The choice of PFS and OS as outcome measures aligns with survival metrics being evaluated as part of the Core Outcome Research Measures in Anal Cancer (CORMAC) initiative and multi-centre prospective anal carcinoma radiotherapy trials being carried out at present [35, 36]. In prospective studies, this can be partially negated by having defined regular follow-up for all patients; however, this is not possible in retrospective series. The use of a binary cutoff value, for example 5-year PFS, would allow for a window for an event to occur meaning the need for accurate dating of recurrence or relapse is not as vital. However, this would limit the number of patients who met the minimum follow-up time and therefore limit the number of events; for this reason, a time-censored outcome was used in this study. In terms of OS, the time to death is more reliable and therefore could explain why in general the models for OS performed better, but it is based on all-cause mortality, and it could be argued some causes or mortality cannot be predicted on PET-CT.
Conclusion
This study has demonstrated with external testing that a combination of age and MTV show potential in predicting OS and PFS in ASCC patients. The predictive ability of PET-CT-derived radiomic features diminished when the influence of MTV was accounted for.
Abbreviations
- ASCC:
-
Anal squamous cell carcinoma
- AtomCAT:
-
Anal cancer Treatment Outcome Modelling with Computer Aided Theragnostics
- CNFT:
-
Christie NHS Foundation Trust
- CORMAC:
-
Core Outcome Research Measures in Anal Cancer
- ESMO:
-
European Society for Medical Oncology
- FDG:
-
2-Deoxy-2-[fluorine-18]fluoro-d-glucose
- GLCM:
-
Grey level co-occurrence matrix
- GLRLM:
-
Grey level run length matrix
- GLSZM:
-
Grey level size zone matrix
- HPV:
-
Human papilloma virus
- HR:
-
Hazard ratios
- ln-ROI:
-
Lymph-node ROI
- LRF:
-
Loco-regional failure
- L-SUVmean :
-
Mean liver standardised uptake value
- LTHT:
-
Leeds Teaching Hospitals NHS Trust
- MTV:
-
Metabolic tumour volume
- NGTDM:
-
Neighbouring grey tone difference matrix
- OS:
-
Overall survival
- PCA:
-
Principal component analysis
- PET-CT:
-
Positron emission tomography/computed tomography
- PFS:
-
Progression-free survival
- PH:
-
Proportional hazards
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
- SRF:
-
Survival Random Forest
- SSVM:
-
Survival Support Vector Machine
- t-ROI:
-
Primary tumour ROI
- ukCAT:
-
UK Computer Aided Theragnostics
References
Wilkinson JR, Morris EJA, Downing A et al (2014) The rising incidence of anal cancer in England 1990–2010: a population-based study. Color Dis 16:234–239. https://doi.org/10.1111/codi.12553
Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A (2017) International trends in anal cancer incidence rates. Int J Epidemiol 46:924–938. https://doi.org/10.1093/ije/dyw276
Heer E, Hackl M, Ferlitsch M, Waldhoer T, Yang L (2020) Trends in incidence of anal cancer in Austria, 1983–2016. Wien Klin Wochenschr 132:438–443. https://doi.org/10.1007/s00508-020-01622-z
James RD, Glynne-Jones R, Meadows HM et al (2013) Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol 14:516–524. https://doi.org/10.1016/S1470-2045(13)70086-X
UKCCCR Anal Cancer Trial Working Party (1996) Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UK Co-ordinating Committee on Cancer Research. Lancet 348(9034):1049–54
Bartelink H, Roelofsen F, Eschwege F et al (1997) Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 15:2040–2049
Flam M, John M, Pajak TF et al (1996) Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 14:2527–2539
Sekhar H, Malcomson L, Kochhar R et al (2020) Temporal improvements in loco-regional failure and survival in patients with anal cancer treated with chemo-radiotherapy: treatment cohort study (1990–2014). Br J Cancer 122:749–758. https://doi.org/10.1038/s41416-019-0689-x
Goh V, Gollub FK, Liaw J et al (2010) Magnetic resonance imaging assessment of squamous cell carcinoma of the anal canal before and after chemoradiation: Can MRI predict for eventual clinical outcome? Int J Radiat Oncol Biol Phys 78:715–721. https://doi.org/10.1016/j.ijrobp.2009.08.055
Glynne-Jones R, Nilsson PJ, Aschele C et al (2014) Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:10–20. https://doi.org/10.1016/j.radonc.2014.04.013
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762. https://doi.org/10.1038/nrclinonc.2017.141
Giraud N, Sargos P, Leduc N, Saut O, Vuoug T, Vendrely V (2020) Radiomics and machine learning in anal squamous cell carcinoma: a new step for personalized medicine? Appl Sci 10(6):1988. https://doi.org/10.3390/app10061988
Brown PJ, Zhong J, Frood R et al (2019) Prediction of outcome in anal squamous cell carcinoma using radiomic feature analysis of pre-treatment FDG PET-CT. Eur J Nucl Med Mol Imaging 46:2790–2799. https://doi.org/10.1007/s00259-019-04495-1
Song J, Yin Y, Wang H, Chang Z, Liu Z, Cui L (2020) A review of original articles published in the emerging field of radiomics. Eur J Radiol 127:108991. https://doi.org/10.1016/j.ejrad.2020.108991
Nioche C, Orlhac F, Boughdad S, Reuze S, Goya-Outi J, Robert C et al (2018) Lifex: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789
Frood R, Clark M, Burton C et al (2022) Discovery of pre-treatment FDG PET/CT-derived radiomics-based models for predicting outcome in diffuse large B-cell lymphoma. Cancers (Basel) 14(7):1711. https://doi.org/10.3390/cancers14071711
Frood R, Burton C, Tsoumpas C et al (2021) Baseline PET/CT imaging parameters for prediction of treatment outcome in Hodgkin and diffuse large B cell lymphoma: a systematic review. Eur J Nucl Med Mol Imaging 48(10):3198–3220. https://doi.org/10.1007/s00259-021-05233-2
Boktor RR, Walker G, Stacey R, Gledhill S, Pitman AG (2013) Reference range for intrapatient variability in blood-pool and liver SUV for 18F-FDG PET. J Nucl Med 54:677–682. https://doi.org/10.2967/jnumed.112.108530
Ligero M, Jordi-Ollero O, Bernatowicz K et al (2021) Minimizing acquisition-related radiomics variability by image resampling and batch effect correction to allow for large-scale data analysis. Eur Radiol 31:1460–1470. https://doi.org/10.1007/s00330-020-07174-0
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The Image Biomarker Standardization Initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295(2):328–338. https://doi.org/10.1148/radiol.2020191145
Bazan JG, Koong AC, Kapp DS et al (2013) Metabolic tumor volume predicts disease progression and survival in patients with squamous cell carcinoma of the anal canal. J Nucl Med 54:27–32. https://doi.org/10.2967/jnumed.112.109470
Rusten E, Rekstad BL, Undseth C et al (2019) Anal cancer chemoradiotherapy outcome prediction using 18F-fluorodeoxyglucose positron emission tomography and clinicopathological factors. Br J Radiol 92:11–13. https://doi.org/10.1259/bjr.20181006
Jones MP, Hruby G, Metser U et al (2019) FDG-PET parameters predict for recurrence in anal cancer - results from a prospective, multicentre clinical trial. Radiat Oncol 14:1–6. https://doi.org/10.1186/s13014-019-1342-9
Braun LH, Reinert CP, Zips D, Nikolaou K, Pfannenberg C, Gani C (2020) Treatment outcome after radiochemotherapy in anal cancer patients staged with 18F-FDG-PET-CT. Clin Transl Radiat Oncol 24:83–87. https://doi.org/10.1016/j.ctro.2020.06.008
Arshad MA, Gitau S, Tam H et al (2021) Optimal method for metabolic tumour volume assessment of cervical cancers with inter-observer agreement on [18F]-fluoro-deoxy-glucose positron emission tomography with computed tomography. Eur J Nucl Med Mol Imaging 48(6):2009–2023. https://doi.org/10.1007/s00259-020-05136-8
van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B (2020) Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 11:91. https://doi.org/10.1186/s13244-020-00887-2
Spadarella G, Stanzione A, Akinci D’Antonoli T et al (2023) Systematic review of the radiomics quality score applications: an EuSoMII Radiomics Auditing Group Initiative. Eur Radiol 33:1884–1894. https://doi.org/10.1007/s00330-022-09187-3
Sanduleanu S, Woodruff HC, de Jong EEC et al (2018) Tracking tumor biology with radiomics: a systematic review utilizing a radiomics quality score. Radiother Oncol 127:349–360. https://doi.org/10.1016/j.radonc.2018.03.033
Piñeiro-Fiel M, Moscoso A, Pubul V, Ruibal Á, Silva-Rodríguez J, Aguiar P (2021) A systematic review of pet textural analysis and radiomics in cancer. Diagnostics 11:1–29. https://doi.org/10.3390/diagnostics11020380
Theophanous S, Lønne PI, Choudhury A et al (2022) Development and validation of prognostic models for anal cancer outcomes using distributed learning: protocol for the international multi-centre atomCAT2 study. Diagn Progn Res 4;6(1):14
Welch ML, McIntosh C, Haibe-Kains B et al (2019) Vulnerabilities of radiomic signature development: the need for safeguards. Radiother Oncol 130:2–9. https://doi.org/10.1016/j.radonc.2018.10.027
Kumar V, Gu Y, Basu S et al (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30:1234–1248. https://doi.org/10.1016/j.mri.2012.06.010
Vickers AJ, Van Calster B, Steyerberg EW (2016) Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 352:i6. https://doi.org/10.1136/bmj.i6
Fallowfield LJ, Fleissig A (2012) The value of progression-free survival to patients with advanced-stage cancer. Nat Rev Clin Oncol 9:41–47. https://doi.org/10.1038/nrclinonc.2011.156
Renehan AG, Muirhead R, Berkman L, McParland L, Sebag-Montefiore D (2019) Early stage anal margin cancer: towards evidence-based management. Colorectal Dis 21:387–391
Fish R, Sanders C, Adams R et al (2018) A core outcome set for clinical trials of chemoradiotherapy interventions for anal cancer (CORMAC): a patient and health-care professional consensus. Lancet Gastroenterol Hepatol 3(12):865–873. https://doi.org/10.1016/S2468-1253(18)30264-4
Acknowledgements
The authors would also like to acknowledge Cancer Research UK funding for the Leeds Radiotherapy Research Centre of Excellence (RadNet), grant number C19942/A28832.
Funding
R Frood received salary support from Innovate UK, A Scarsbrook received salary support from Innovate UK and Cancer Research UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof A Scarsbrook.
Conflict of interest
A Scarsbrook is an Associate Editor of European Radiology and a member of the Editorial Board for the journal. They have not taken part in the review or selection process of this article.
Statistics and biometry
One of the authors (Dr H Mistry) has significant statistical expertise.
Informed consent
All patients included within the study gave prospective consent at the time of imaging for their anonymised FDG PET-CT imaging data to be used in research and service development projects.
Ethical approval
As this is a retrospective study, which does not involve patient contact or the alteration of treatment, following discussion with the Research and Innovation Department at Leeds Teaching Hospitals NHS Trust (LTHT), it was agreed that this represented a service improvement project and requirement for formal ethics committee approval was waived for LTHT data collection. Data collection at Christie NHS Foundation Trust (CNFT) was approved by the UK Computer Aided Theragnostics (ukCAT) ethics committee.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported. The dataset (187 patients) from Leeds has been used for a radiomics-based prediction model which has been published previously—Brown PJ, Zhong J, Frood R, et al (2019) Prediction of outcome in anal squamous cell carcinoma using radiomic feature analysis of pre-treatment FDG PET-CT. Eur J Nucl Med Mol Imaging 46: 2790–2799. However, this work looks at a range of different prediction models using the same data set representing new original research. In addition, the current study includes external validation with a large independent dataset not previously published.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frood, R., Mercer, J., Brown, P. et al. Training and external validation of pre-treatment FDG PET-CT-based models for outcome prediction in anal squamous cell carcinoma. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10340-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10340-9