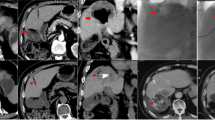
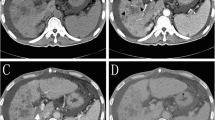
Abstract
Objectives
To determine the safety and efficacy of transcatheter arterial chemoembolization with CalliSpheres® beads loaded with arsenic trioxide (CBATO-TACE) in the first-line treatment of patients with large (5 cm ≤ maximum diameter < 10 cm) or huge (maximum diameter ≥ 10 cm) hepatocellular carcinoma (HCC).
Methods
Patients were randomly allocated to the CBATO-TACE group and the conventional transcatheter arterial chemoembolization (cTACE) group. The primary endpoint was progression-free survival (PFS). The secondary endpoint was overall survival (OS), treatment response, and treatment-related adverse events (TRAEs). The extrahepatic collateral arteries, liver function, and liver fibrosis after the first TACE were also evaluated.
Results
From September 2018 to September 2020, a total of 207 patients who underwent TACE were consecutively enrolled in this study. The median PFS was 9.5 months (range: 8.0 – 11.0) in the CBATO group, which was significantly longer than that in the cTACE group (6.0 months, range: 4.0–6.0) (p < 0.0001). Patients in the CBATO group had a median OS of 22 months (range: 20.0 – 27.0) compared with 16 months (range: 15.0 – 20.0) in the cTACE group (p = 0.0084). The most common TRAEs were fever (p = 0.043), and nausea and vomiting (p = 0.002), which were more observed in the cTACE group. In addition, the progressive disease time, pulmonary metastasis rate (p = 0.01), the mean number of extrahepatic collateral arteries (p = 0.01), and average number of TACE sessions (p = 0.025) were significantly decreased in the CBATO group.
Conclusions
CBATO-TACE achieved better therapeutic outcomes and similar safety profile compared to cTACE in large or huge HCC patients. Furthermore, CBATO-TACE was able to reduce extrahepatic collateral arteries production and extrahepatic lung metastasis.
Clinical relevance statement
Our study showed that CalliSpheres® beads loaded with arsenic trioxide (CBATO-TACE) were effective and safe for the treatment of large and giant HCC. In addition, CBATO-TACE can reduce lateral hepatic branch artery formation and extrahepatic pulmonary metastasis, which provides a new treatment approach for unresectable HCC.
Key Points
• We compare long-term efficacy and safety of transcatheter arterial chemoembolization with CalliSpheres® beads loaded with arsenic trioxide (CBATO-TACE) and conventional transcatheter arterial chemoembolization (cTACE) in patients with large (5 cm ≤ maximum diameter < 10 cm) or huge HCC (maximum diameter ≥ 10 cm).
• Compared with cTACE, CBATO-TACE significantly improved therapeutic outcomes, overall survival, and progression-free survival in patients with large or huge HCC. The safety assessment suggested that CBATO-TACE is a safe treatment that improves the quality of life and has good treatment adherence.




Similar content being viewed by others
Abbreviations
- AEs:
-
Adverse events
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- ATO:
-
Arsenic trioxide
- BCr:
-
Blood creatinine
- BUN:
-
Blood urea nitrogen
- CBATO-TACE:
-
CalliSpheres® beads loaded with arsenic trioxide
- CR:
-
Complete remission
- cTACE:
-
Conventional transcatheter arterial chemoembolization
- DCR:
-
Disease control rate
- DEB-TACE:
-
Drug-eluting bead TACE
- EHC:
-
Extrahepatic collateral
- HCC:
-
Hepatocellular carcinoma
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RBC:
-
Red blood cell
- WBC:
-
White blood cell
References
Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380(15):1450–1462
Liu B, Huang JW, Li Y et al (2015) Arsenic trioxide transarterial chemoembolization with and without additional intravenous administration of arsenic trioxide in unresectable hepatocellular carcinoma with lung metastasis: a single-blind, randomized trial. J Cancer Res Clin Oncol 141(6):1103–1108
Li Z, Chen Q, Zhang W, Si G, Li J (2021) Jiao D et al Efficacy and safety of the arsenic trioxide/lipiodol emulsion in the transcatheter arterial chemoembolization combined with apatinib in the treatment of advanced hepatocellular carcinoma. Can J Gastroenterol Hepatol 2021:5565793
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF (2016) Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology 64(1):106–116
Li C, Wang MD, Lu L et al (2019) Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (>/= 10 cm): a multicenter propensity matching analysis. Hepatol Int 13(6):736–747
Wakayama K, Kamiyama T, Yokoo H et al (2017) Huge hepatocellular carcinoma greater than 10 cm in diameter worsens prognosis by causing distant recurrence after curative resection. J Surg Oncol 115(3):324–329
Pawlik TM, Delman KA, Vauthey JN et al (2005) Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 11(9):1086–1092
Miyayama S, Kikuchi Y, Yoshida M et al (2019) Outcomes of conventional transarterial chemoembolization for hepatocellular carcinoma >/=10 cm. Hepatol Res 49(7):787–798
Sanz MA, Fenaux P, Tallman MS et al (2019) Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood 133(15):1630–1643
Kutny MA, Alonzo TA, Abla O et al (2022) Assessment of arsenic trioxide and all-trans retinoic acid for the treatment of pediatric acute promyelocytic leukemia: a report from the Children’s Oncology Group AAML1331 Trial. JAMA Oncol 8(1):79–87
Wang X, Cao L, Wu J et al (2021) Exploring the mechanisms of arsenic trioxide (Pishuang) in hepatocellular carcinoma based on network pharmacology. Evid Based Complement Alternat Med. 2021:5773802
Ma Y, Wang J, Liu L et al (2011) Genistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: role of Akt and nuclear factor- kappaB. Cancer Lett 301(1):75–84
Sadaf N, Kumar N, Ali M, Ali V, Bimal S, Haque R (2018) Arsenic trioxide induces apoptosis and inhibits the growth of human liver cancer cells. Life Sci 205:9–17
Zhang F, Zhang CM, Li S et al (2018) Low dosage of arsenic trioxide inhibits vasculogenic mimicry in hepatoblastoma without cell apoptosis. Mol Med Rep 17(1):1573–2158
Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL, Yang CH (2007) Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Invest New Drugs 25(1):77–84
Vesselle G, Quirier-Leleu C, Velasco S et al (2016) Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol 26(6):1640–1648
Song MJ, Chun HJ, Song DS et al (2012) Comparative study between doxorubicin- eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 57(6):1244–1250
Duan XH, Ju SG, Han XW et al (2020) Arsenic trioxide-eluting CalliSpheres beads is more effective and equally tolerant compared with arsenic trioxide/lipiodol emulsion in the transcatheter arterial chemoembolization treatment for unresectable hepatocellular carcinoma patients. Eur Rev Med Pharmacol Sci 24(3):1468–1480
Duan X, Li H, Han X et al (2020) Antitumor properties of arsenic trioxide-loaded CalliSpheres® microspheres by transarterial chemoembolization in VX2 liver tumor rabbits: suppression of tumor growth, angiogenesis, and metastasis and elongation of survival. Am J Transl Res 12(9):5511–5524
Health and Family Planning Commission of the People’s Republic of China, Medical Affairs and Medical Administration Bureau (2017) Diagnosis and treatment specifications for primary liver cancer (2017 version) [J]. Chin J Gastroenterol 16 (7):13
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
Liu S, Li H, Guo L et al (2019) Tumor size affects efficacy of adjuvant transarterial chemoembolization in patients with hepatocellular carcinoma and microvascular invasion. Oncologist 24(4):513–520
Tsoulfas G, Mekras A, Agorastou P, Kiskinis D (2012) Surgical treatment for large hepatocellular carcinoma: does size matter? ANZ J Surg 82(7–8):510–517
Zhong JH, Rodriguez AC, Ke Y, Wang YY, Wang L, Li LQ (2015) Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine (Baltimore) 94(3):e396
Chang YJ, Chung KP, Chang YJ, Chen LJ (2016) Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg 103(11):1513–1520
Huang YH, Wu JC, Chen SC et al (2006) Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther 23(1):129–135
Sun J, Zhou G, Xie X et al (2020) Efficacy and safety of drug-eluting beads transarterial chemoembolization by CalliSpheres((R)) in 275 hepatocellular carcinoma patients: results from the Chinese CalliSpheres((R)) Transarterial Chemoembolization in Liver Cancer (CTILC) study. Oncol Res 28(1):75–94
Du X, Chen D, Lin Z et al (2019) Efficacy of apatinib in advanced hepatocellular carcinoma with lung metastasis: a retrospective, multicenter study. J Buon 24(5):1956–1963
Hu HT, Yao QJ, Meng YL et al (2017) Arsenic trioxide intravenous infusion combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with pulmonary metastasis: long-term outcome analysis. J Gastroenterol Hepatol 32(2):295–300
Wang H, Liu Y, Wang X et al (2015) Randomized clinical control study of locoregional therapy combined with arsenic trioxide for the treatment of hepatocellular carcinoma. Cancer 121(17):2917–2925
Duan XH, Li H, Ren JZ et al (2019) Hepatic arterial chemoembolization with arsenic trioxide eluting CalliSpheres microspheres versus lipiodol emulsion: pharmacokinetics and intratumoral concentration in a rabbit liver tumor model. Cancer Manag Res 11:9979–9988
Duan X, Li H, Han X et al (2020) Antitumor properties of arsenic trioxide-loaded CalliSpheres((R)) microspheres by transarterial chemoembolization in VX2 liver tumor rabbits: suppression of tumor growth, angiogenesis, and metastasis and elongation of survival. Am J Transl Res 12(9):5511–5524
Bruix J, Sherman M, American Association for the Study of Liver D (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022
Moustafa AS, Abdel Aal AK, Ertel N, Saad N, DuBay D, Saddekni S (2017) Chemoembolization of hepatocellular carcinoma with extrahepatic collateral blood supply: anatomic and technical considerations. Radiographics 37(3):963–977
Lokken RP, Fidelman N, Kolli KP, Kerlan RK Jr (2016) Safety and efficacy of doxorubicin drug-eluting embolic chemoembolization of hepatocellular carcinoma supplied by extrahepatic collateral arteries. J Vasc Interv Radiol 27(11):1698–1704
Funding
This work was supported by the National Natural Science Foundation of China (No. U2004119), the National Natural Science Foundation of Henan Province (No. 202300410361), and the Beijing Medical Award Foundation (No. YXJL-2021–0072-0349).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xinwei Han.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR1800018418).
Study subjects or cohorts overlap
None.
Methodology
• Prospective
• Randomized controlled trial
• Performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, X., Li, H., Chen, P. et al. Transcatheter arterial chemoembolization using CalliSpheres beads loaded with arsenic trioxide for unresectable large or huge hepatocellular carcinoma: a prospective study. Eur Radiol 34, 1258–1267 (2024). https://doi.org/10.1007/s00330-023-10097-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10097-1