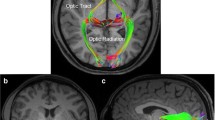
Abstract
Objectives
To investigate whether morphological changes after surgery and delta-radiomics of the optic chiasm obtained from routine MRI could help predict postoperative visual recovery of pituitary adenoma patients.
Methods
A total of 130 pituitary adenoma patients were retrospectively enrolled and divided into the recovery group (n = 87) and non-recovery group (n = 43) according to visual outcome 1 year after endoscopic endonasal transsphenoidal surgery. Morphological parameters of the optic chiasm were measured preoperatively and postoperatively, including chiasmal thickness, deformed angle, and suprasellar extension. Delta-radiomics of the optic chiasm were calculated based on features extracted from preoperative and postoperative coronal T2-weighted images, followed by machine learning modeling using least absolute shrinkage and selection operator wrapped with support vector machine through fivefold cross-validation in the development set. The delta-radiomic model was independently evaluated in the test set, and compared with the combined model that incorporated delta-radiomics, significant clinical and morphological parameters.
Results
Postoperative morphological changes of the optic chiasm could not significantly be used as predictors for the visual outcome. In contrast, the delta-radiomics model represented good performances in predicting visual recovery, with an AUC of 0.821 in the development set and 0.811 in the independent test set. Moreover, the combined model that incorporated age and delta-radiomics features of the optic chiasm achieved the highest AUC of 0.841 and 0.840 in the development set and independent test set, respectively.
Conclusions
Our proposed machine learning models based on delta-radiomics of the optic chiasm can be used to predict postoperative visual recovery of pituitary adenoma patients.
Clinical relevance statement
Our delta-radiomics-based models from MRI enable accurate visual recovery predictions in pituitary adenoma patients who underwent endoscopic endonasal transsphenoidal surgery, facilitating better clinical decision-making and ultimately improving patient outcomes.
Key Points
• Prediction of the postoperative visual outcome for pituitary adenoma patients is important but challenging.
• Delta-radiomics of the optic chiasm after surgical decompression represented better prognostic performances compared with its morphological changes.
• The proposed machine learning models can serve as novel approaches to predict visual recovery for pituitary adenoma patients in clinical practice.
Similar content being viewed by others
Introduction
Pituitary adenomas are the most prevalent sellar region neoplasm [1, 2]. Patients with pituitary adenoma may suffer from significant visual dysfunctions as the location of tumor growth is in proximity to critical structures for vision, especially referred to as optic chiasm. Compression of the optic chiasm can lead to visual morbidity and subsequent compromised quality of life [3,4,5]. It is also one of the main indicators for therapeutic intervention [6, 7]. Endoscopic endonasal transsphenoidal surgery (EETS) has become one of the predominant choices for tumor resection, which has shown promising results regarding tumor resection rates and postoperative visual improvement [8].
Identifying prognostic factors of favorable visual outcomes is of major importance, as this can aid clinical decision-making about the benefits of surgical intervention. For patients at high risk for persistent visual impairment, timely initiation of operation and referral for visual rehabilitation after surgery are crucial to preserve visual function and improve quality of life [9, 10]. Previous researchers attempted to identify predictive factors that correlate with the postoperative visual outcome, including tumor size, age, preoperative visual field, and retinal nerve fiber layer (RNFL) thickness [11,12,13,14]. Whereas, the results are still controversial as unquantified outcomes, small sample sizes, and partial predictors constitute the limitations of these various types of research. Besides, some parameters they used are either expensive or not easily obtained from routine clinical practice [15,16,17]. A more practical, convenient method is required to meet the need for individualized patient management strategies.
The noninvasive quantification of medical images is a novel and promising field of research referred to as radiomics. By retrieving high-throughput information from images, radiomics techniques aim at providing personalized medicine approaches by leveraging big-data analytics [18, 19]. With the extraction of thousands of numeric features and careful feature selections, machine-learning classifiers can be designed and trained to answer some crucial questions beyond naked-eyes’ assessment, such as patient outcome prediction [20]. Nowadays, for neurosurgeons and neuroradiologists, who might need most assistance from precise prognostic prediction, the MR scan is still the standard examination for both diagnosis and follow-up of pituitary adenoma patients [21, 22]. Morphological assessment of optic chiasm on MRI is the primary choice to evaluate the compression status of the optic chiasm and visual outcome of patients in clinical work [23, 24]. Moreover, visual MRI evaluation before and after surgery can highlight a series of morphologic recovery of the optic chiasm associated with functional recovery that may be quantified with radiomics features [25]. Considering the visual recovery of pituitary adenoma patients after surgery is a dynamic process, the data involving radiomics feature changes (also referred to as delta-radiomics) of the optic chiasm during surgical decompression would be more informative. Delta-radiomics is a method that analyzes the relative net changes in radiomics parameters using a set of consecutive images to provide a quantitative assessment of the underlying histopathological changes. In recent various types of research, delta-radiomics has been utilized to predict the treatment response and prognosis in many diseases and represents promising performances [26,27,28,29]. Therefore, in the current research, with routine MRI, we aimed to investigate if changes in traditional morphological parameters and radiomics features of the optic chiasm after surgical decompression could help predict the visual outcome of pituitary adenoma patients who underwent EETS.
Materials and methods
Patient population
This retrospective study was approved by the institutional review board, and written informed consent was waived (2021-S-851). The inclusion criteria were patients who (1) underwent EETS in curative intent between March 2018 and January 2021; (2) with pathologically proven pituitary adenoma; (3) presence of tumor-related chiasmal compression and visual field (VF) defects before surgery; (4) with sellar MRI within two weeks preoperatively and within three days postoperatively; (5) with VF testing at 1 year after operation. The exclusion criteria encompassed: (1) recurrent pituitary adenoma (n = 13); (2) history of radiation before or after surgery (n = 10); (3) poor image quality of MRI with artifacts (n = 5); (4) unrecognizable optic chiasm caused by severe tumor compression (n = 6); (5) history of any other ophthalmological diseases like cataract or glaucoma (n = 4); (6) unreliable VF testing, defined as > 33% false negative responses or false positives responses (n = 2); (7) unexpected postoperative VF deterioration (more than a 10 dB decrease in mean deviation) implying surgical injury (n = 2) [14]. The flow chart of patient selection was shown in Fig. 1. Furthermore, we collected the clinical characteristics of qualified patients, including age, gender, histological subtype, and Ki-67 index of tumors.
Ophthalmological assessment
The VF of all qualified patients were assessed with standard automated perimetry (OCTOPUS 900, Haag-Streit Inc; Humphrey Field Analyzer, Carl Zeiss Meditec Inc) preoperatively and postoperatively. A mean deviation (MD) worse than − 3.0 dB was regarded as the VF defect. Patients were divided into two groups according to the results of the VF testing 1 year after surgery: the recovery group (postoperative MD ≥ − 3.0 dB) and the non-recovery group (postoperative MD < − 3.0 dB), as suggested by previous research [12, 13, 30].
Image acquisition and morphologic evaluation
The MR images within two weeks before surgery and within three days after surgery were exported for all eligible patients. Considering coronal slices of sellar MRI best demonstrate chiasmal compression and the boundary of the optic chiasm is clearly displayed in T2-weighted imaging (T2WI), coronal T2WI were used for the morphologic evaluation and segmentation of the optic chiasm. The acquisition parameters for T2WI were summarized in Supplementary Materials 1.
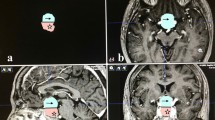
Morphologic parameters of the optic chiasm, including chiasmal thickness, chiasmal deformed angle, and chiasmal suprasellar extension, were measured preoperatively and postoperatively by two neuroradiologists together blinded to patient information (Fig. 2). The chiasmal thickness was measured as the distance between the upper and lower margin of the optic chiasm at the maximal deflection of all slices. The chiasmal deformed angle was defined as the minimal angle created by drawing lines from two sides of the optic chiasm to the midline along the lower margin of all slices. The chiasmal suprasellar extension was defined as the maximal perpendicular distance between the lower margin of the optic chiasm and a reference line at the level of the upper surface of bilateral internal carotid arteries within the cavernous sinus. The absolute change and relative change rate of the above morphologic parameters of the optic chiasm after surgical decompression were also calculated.
Image preprocessing and radiomics feature extraction
Before image preprocessing, all MR images were anonymized, and all private information and protected health information were blinded from DICOM files. Image preprocessing included image resampling of T2WI to 1 × 1 × 1 mm3 voxel resolution and N4ITK bias correction.
Regions of interest (ROI) of optic chiasm were delineated slice by slice by two neuroradiologists separately using the open-source software ITK-SNAP v3.8.0 [31]. The discontinuous portions anterior or posterior to the optic chiasm, regarded as optic nerves or optic tracts, were excluded in ROI delineation. By using the open-source package PyRadiomics v3.0, a total number of 851 quantitative imaging features were retrieved from ROI to decode the radiographic phenotype following the Imaging Biomarker Standardization Initiative (IBSI) guideline [32]. The bin width was set to 5 according to the recommendation of PyRadiomics (https://github.com/AIM-Harvard/pyradiomics). Radiomic features included shape features (n = 14), histogram features (n = 18), texture features (n = 75), and wavelet-based features (n = 744). Intraclass correlation coefficients (ICC) of features extracted by two neuroradiologists were calculated to exclude features with significant intra-observer variations. Only radiomics features with ICC > 0.8 in both preoperative and postoperative image sets were considered reproducible and collected for further analysis. The delta-radiomics features depicting the relative change in radiomics features of the optic chiasm obtained from preoperative (Featurepre) and postoperative (Featurepost) images were calculated using the following formula: ΔFeature = (Featurepost − Featurepre) / Featurepre.
Delta-radiomics machine learning modeling
The whole dataset (n = 130) was randomly partitioned into the development set (n = 91) and independent test set (n = 39) on the proportion of 7: 3 using stratified sampling. Our model was trained based on the delta-radiomics features in the development set by the least absolute shrinkage and selection operator (LASSO) feature selection method, wrapped with a linear support vector machine (SVM) classifier through a five-fold cross-validation approach. The model with the least overfitting and closest performance to the average performance of all five models was selected [33]. Then the model was applied in the test set which was kept untouched during the model training to obtain the generalizability of our predictive model for independent patients. The area under the receiver operating characteristic curve (AUC), F1-score, accuracy, specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) were used to evaluate the performance of predictive models. The calibration of the model was evaluated with calibration plots. Decision curve analysis was utilized to assess the clinical usefulness of the model [34]. All modeling algorithms were conducted using R 3.6.3. The overall workflow of this study was illustrated in Fig. 3.
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics 21 (IBM Corp.) and R 3.6.3. Means and standard deviations are used for the representation of continuous variables, and frequencies and percentages are used for categorical variables. Analysis was carried out using t-test, Mann–Whitney U-test, and chi-square test, as appropriate. The DeLong test was used to compare AUCs of different predictive models [35]. p < 0.05 was deemed statistically significant.
Results
Clinical characteristics
The cohort included 130 patients (74 females, mean age: 49.2 ± 13.3 years old), of which 87 (66.9%) experienced complete VF recovery 1 year after EETS. The average age of patients in the recovery group was significantly younger than that in the non-recovery group (47.1 ± 12.2 and 53.4 ± 14.7 years, respectively; p = 0.010). The preoperative MD of patients in the recovery and non-recovery groups was − 7.6 ± 6.0 dB and − 9.2 ± 6.3 dB, respectively (p = 0.154). The maximal tumor diameter was 23.7 ± 6.9 mm in the recovery group and 24.9 ± 7.5 mm in the non-recovery group (p = 0.349). No significant differences were observed between the recovery and non-recovery groups in terms of gender (p = 0.578), histologic subtype (p = 0.890), and Ki-67 index (p = 0.061). Detailed clinical characteristics of enrolled patients were provided in Table 1. The MRI scanner distribution between the recovery and non-recovery groups was provided in Supplementary Materials 2.
Morphological parameters of the optic chiasm
There were no significant differences between the recovery group and the non-recovery group regarding preoperative or postoperative morphological parameters of the optic chiasm, including chiasmal thickness, chiasmal deformed angle, and chiasmal suprasellar extension (Fig. 4).
Preoperative and postoperative morphological parameters of the optic chiasm in the recovery group and non-recovery group, including chiasmal thickness (A), chiasmal deformed angle (B), and chiasmal suprasellar extension (C). All three morphological parameters of the optic chiasm showed significant changes after surgical decompression, while neither preoperative nor postoperative morphological parameters showed significant differences between the recovery and non-recovery group. ***: p < 0.001; **: p < 0.01; ns: not significant
Compared with preoperative morphological parameters, the thickness of the optic chiasm after surgical decompression was significantly increased (p < 0.001), the chiasmal deformed angle was also significantly increased (p < 0.001) and chiasmal suprasellar extension was significantly reduced (p < 0.001) (Table 2). Specifically, chiasmal thickness in the recovery group and the non-recovery group was increased by 0.3 mm (40.5%) and 0.3 mm (34.2%), respectively. The chiasmal deformed angle in the recovery and non-recovery groups was increased by 16.8 degrees (14.4%) and 14.6 degrees (12.6%), respectively. Chiasmal suprasellar extension in the recovery and non-recovery groups was decreased by 2.6 mm (14.3%) and 2.5 mm (13.2%), respectively. However, neither the absolute nor relative changes in morphological parameters of the optic chiasm after surgical decompression differed significantly between the recovery group and the non-recovery group (Table 3).
Radiomics feature selection and evaluation
Following radiomics extraction and delta-radiomics features calculation, a total of six delta-radiomics features were selected (Supplementary Materials 3). Details regarding the feature selection process were provided in Supplementary Materials 4. The association between values of the six delta-radiomics features and the postoperative visual recovery was illustrated in Supplementary Materials 5.
Furthermore, we investigated the prognostic potentials of the presurgical values of the above six parameters. One parameter (Wavelet-LLL-GLSZM-Zone Variance) showed a significant difference between the recovery and non-recovery group and could be taken as the independent predictor (odds ratio = 1.580, p = 0.046) for visual recovery after correcting for the age by multivariate logistic regression analysis (Supplementary Materials 6). Receiver operating characteristic analysis showed that its AUC value was 0.653 (95% confidence interval [CI]: 0.548–0.759).
Performance of delta-radiomics models
The predictive model for the visual recovery was constructed with the above six delta-radiomics features. With this delta-radiomics model, an AUC of 0.821 (95% CI: 0.729–0.914), with associated F1-score, sensitivity, and specificity of 0.793, 0.721, and 0.800, respectively, was obtained in the development set. This model was evaluated in the independent test set, achieving an AUC of 0.811 (95% CI: 0.692–0.930), with an F1-score of 0.889, sensitivity of 0.923, and specificity of 0.692, respectively.
Considering age was the only clinical parameter with a significant difference between the recovery and non-recovery groups, its prognostic value for VF recovery was also investigated. AUC values of age in the development set and the independent test set were 0.641 and 0.655, respectively. The DeLong test showed that compared with the age, the delta-radiomics model represented statistically better predictive performances for the VF outcome in both the development set (p = 0.014) and the independent test set (p = 0.040). Furthermore, the combined model that incorporated age and 6 delta-radiomics features performed commendably in predicting the VF outcome, evidenced by AUC values of 0.841 (95% CI: 0.750–0.932) and 0.840 (95% CI: 0.733–0.947) in the development set and the independent test set, respectively. According to the DeLong test, predictive performances of the combined model were also statistically better than age in both the development set (p = 0.001) and the independent test set (p = 0.029). Besides, the combined model also represented comparable performance with predictors in previous studies (Supplementary Material 7). However, there were no significant differences regarding AUCs between the delta-radiomic model and the combined model in the development set (p = 0.364) and the independent test set (p = 0.497). The detailed predictive performances of different models were summarized in Table 4, and their receiver operating characteristic (ROC) curves were shown in Fig. 5.
Receiver operating characteristic curves of different models in predicting the postoperative visual outcome of pituitary adenoma patients in the development set (A) and independent test set (B). The delta-radiomics model and combined model represented higher AUC than the age in both the development and independent test set
The calibration plots showed that the observed probabilities of visual recovery were consistent with the predicted probabilities of the delta-radiomics model and combined model in both the development and independent test set (Fig. 6A, B). Moreover, the decision curves illustrated that both the delta-radiomics model and combined model provided significant improvement in the net benefit compared with the age in the development and independent test set (Fig. 6C, D).
Calibration plots and decision curves of different models in the development set and independent test set. A, B The calibration plots illustrated that the delta-radiomics model and combined model showed good calibration with a closer fit to the diagonal dashed line that represents an ideal evaluation by a perfect model; C, D The decision curves illustrated that our models were clinically available. The black solid line represents all patients with visual recovery, while the black dashed line represents all patients without visual recovery
Discussion
Despite several prognostic factors for visual outcome that have been investigated in previous research, the prediction of postoperative visual recovery for pituitary adenoma patients with a more practical and accurate approach remains a challenge [36]. Changes in morphological parameters and radiomics features of the optic chiasm on MRI after surgical decompression may reveal prognostic insights to predict the VF outcome of pituitary adenoma patients. The present study found that postoperative morphological changes of the optic chiasm could not significantly be used as predictors for the visual outcome. Instead, delta-radiomics of the optic chiasm derived from routine MR images after surgical decompression achieved feasible performances in predicting the visual outcome of pituitary adenoma patients following EETS.
The visual recovery of pituitary adenoma patients following EETS is a long-term and gradually developing process. Early visual improvement occurs immediately after surgical chiasmal decompression that leads to rapid reversal of conduction block, and the improvement of axoplasmic transport and remyelination contribute to sustained visual recovery over weeks to months after surgery [36]. Previous research demonstrated that the early morphological recovery of the optic chiasm after operation may be associated with the functional recovery of pituitary adenoma patients, and postoperative restoration of symmetry of the optic chiasm could suggest sufficient VF recovery [25]. However, the evaluation of chiasmal symmetry on MRI is relatively subjective, and quantitative analysis of the morphological recovery of optic chiasm in pituitary adenoma patients following EETS has never been reported in previous studies. Therefore, in the present study, we utilized a series of imaging parameters to quantitatively analyze early morphological changes of the optic chiasm after surgical decompression and investigated their prognostic values of VF outcome in pituitary adenoma patients. The results indicated that the thickness and deformed angle of the optic chiasm were significantly increased, and its suprasellar extension was significantly reduced within 72 h after EETS. However, these morphological changes, whether in absolute change or relative change rate, were not significantly different between the recovery group and the non-recovery group, indicating that the association between early macroscopic morphological changes of the optic chiasm after operation and functional recovery of pituitary adenoma patients may be relatively inadequate.
Compared with traditional imaging parameters, radiomics has the potential to detect the underlying pathophysiological process beyond the naked eye [18]. Given that the postoperative visual recovery of pituitary adenoma patients is a dynamic process, the delta-radiomics features of the optic chiasm that involved changes in chiasmal microscopic imaging characteristics along with surgical decompression would be more informative. In recent years, delta-radiomics has shown promising performances in predicting disease progression and monitoring treatment response [26,27,28,29]. Since previous researchers suggested the early morphological recovery of the optic chiasm after surgery was associated with long-term visual recovery, we hypothesized that delta-radiomics of the optic chiasm in the early postoperative period may predict the final visual outcome [25, 37]. Therefore, preoperative and early postoperative images were utilized to construct the predictive model. Our results showed that the machine learning model based on delta-radiomics of the optic chiasm within 72 h after surgical decompression represented good performances in predicting the VF recovery of pituitary adenoma patients, suggesting that changes of microscopic image characteristics of the optic chiasm may better reflect functional reversibility and is more relevant to the visual outcome of pituitary adenoma patients compared with macroscopic morphological changes. Six delta-radiomics features were selected as they were correlated with visual outcomes. Specifically, five parameters showed smaller changes in patients with better visual outcomes after surgery, including contrast (a measure of the spatial intensity change and the overall gray level dynamic range) from both original and wavelet images, median (the median gray level intensity within the area), and inverse variance and zone variance (a measure of the variance in the area). The possible speculation is that the more stable microscopic image patterns of the optic chiasm during surgical decompression may imply a better recovery process and visual outcome. Only the larger change of Cluster Shade (a measure of the skewness and uniformity of the gray level co-occurrence matrix) was associated with the postoperative visual recovery. Among the above six parameters, only Contrast from neighboring gray tone difference matrix has been suggested to be related to pathological changes of the optic nerve, like demyelination and axonal loss in optic neuritis patients by previous researchers [38]. Whereas, the specific relationship between the other five radiomic parameters and pathological changes in optic neuropathies has limitedly been explored. Detailed implications of these voxel-wise features to visual function and outcome require more investigations in the future.
Clinical characteristics and parameters obtained from optical coherence tomography (OCT) were commonly used to predict visual outcomes of pituitary adenoma patients with inconsistent results. In our study, increased age was a negative prognostic factor for VF recovery, which was in accordance with previous types of research [39,40,41]. However, the prognostic value of age is relatively limited, and both our delta-radiomics model and combined model significantly outperformed the age in predicting the VF recovery of pituitary adenoma patients. In our study, we explored the prognostic potential of preoperative morphological and radiomics parameters, although their predictive capabilities were not ideal. This could be due to the influence of the surgical procedure on visual outcomes. Combining preoperative and postoperative images may yield more accurate predictions than using only preoperative images. In addition, our combined model also represented competitive predictive performances compared with most developed models in previous studies that usually incorporated OCT-based parameters like RNFL and ganglion cell layer thickness (Supplementary Material 5) [12, 13, 30, 42,43,44]. One study utilized MRI compression grade to evaluate the compression degree of the optic chiasm and combined it with preoperative MD and RNFL thickness to build a predictive model for visual recovery that achieved similar performances (AUC = 0.84) to our models [12]. More importantly, compared with OCT which was not routinely conducted for pituitary adenoma patients in clinical works, our proposed models were constructed using conventional MR images that were easily obtained from routine clinical management of pituitary adenoma patients receiving EETS, indicating that our models could potentially be utilized in clinical practice to assist in predicting the visual outcome in the early postoperative period. For patients who may be predicted to have poor outcomes, timely visual rehabilitation after surgery is needed to preserve visual function as much as possible and improve quality of life.
The present study has several limitations. First, this study is limited by its single-center, retrospective nature with the inherent selection bias. Given the small sample size and wide range of 95% CI of the metrics, the real predictive performance of our delta-radiomic models may not be so optimistic. Future prospective studies are required to validate our model thoroughly in multi-institutional datasets with large cohorts of patients. Second, previously reported prognostic factors obtained from OCT were not considered in this study, as OCT was not routinely performed for pituitary adenoma patients in our center. Future research is warranted to explore whether the incorporation of OCT-based parameters with radiomics could further improve the performance of the predictive model. Third, some cases were excluded as their unrecognizable optic chiasm due to extremely severe compression by tumors. This limitation may affect the model's applicability in real-world clinical scenarios. Fourth, images within three days after the operation were used for delta-radiomics calculation based on the experiences of neurosurgeons, as they urge to predict visual outcomes in a short time after EETS. However, the visual recovery takes place for months and the assessment of recovery takes place 1 year later. More research is required to investigate the appropriate time point for the recovery prediction.
In conclusion, compared with morphological changes of the optic chiasm after surgical decompression, delta-radiomics of the optic chiasm have better prognostic values for visual recovery in pituitary adenoma patients following EETS. Based on delta-radiomics of the optic chiasm derived from routine MR images, we proposed novel machine learning models that are effective in predicting the postoperative visual outcome of pituitary adenoma patients.
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- CI:
-
Confidence interval
- EETS:
-
Endoscopic endonasal transsphenoidal surgery
- IBSI:
-
Imaging Biomarker Standardization Initiative
- ICC:
-
Intraclass correlation coefficients
- LASSO:
-
Least absolute shrinkage and selection operator
- MD:
-
Mean deviation
- NPV:
-
Negative predict value
- OCT:
-
Optical coherence tomography
- PPV:
-
Positive predict value
- RNFL:
-
Retinal nerve fiber layer
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interests
- SVM:
-
Support vector machine
- T2WI:
-
T2-weighted imaging
- VF:
-
Visual field
References
Melmed S, Kaiser UB, Lopes MB et al (2022) Clinical Biology of the Pituitary Adenoma. Endocr Rev 43(6):1003–1037. https://doi.org/10.1210/endrev/bnac010
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol 23(12 Suppl 2):iii1-iii105 https://doi.org/10.1093/neuonc/noab200
Abouaf L, Vighetto A, Lebas M (2015) Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol 76(3):210–219. https://doi.org/10.1016/j.ando.2015.04.006
Andela CD, Scharloo M, Pereira AM, Kaptein AA, Biermasz NR (2015) Quality of life (QoL) impairments in patients with a pituitary adenoma: a systematic review of QoL studies. Pituitary 18(5):752–776. https://doi.org/10.1007/s11102-015-0636-7
Andela CD, Niemeijer ND, Scharloo M et al (2015) Towards a better quality of life (QoL) for patients with pituitary diseases: results from a focus group study exploring QoL. Pituitary 18(1):86–100. https://doi.org/10.1007/s11102-014-0561-1
Lithgow K, Batra R, Matthews T, Karavitaki N (2019) Management of endocrine disease: visual morbidity in patients with pituitary adenoma. Eur J Endocrinol 181(5):R185-r197. https://doi.org/10.1530/eje-19-0349
Okamoto Y, Okamoto F, Hiraoka T, Yamada S, Oshika T (2008) Vision-related quality of life in patients with pituitary adenoma. Am J Ophthalmol 146(2):318–322. https://doi.org/10.1016/j.ajo.2008.04.018
Gong X, Zhuo Y, Yuan H et al (2022) Outcome of endoscopic transsphenoidal surgery for recurrent or residual pituitary adenomas and comparison to non-recurrent or residual cohort by propensity score analysis. Front Endocrinol 13:837025 https://doi.org/10.3389/fendo.2022.837025
Oeverhaus M, Dekowski D, Hirche H, Esser J, Schaperdoth-Gerlings B, Eckstein A (2020) Visual rehabilitation of patients with corneal diseases. BMC Ophthalmol 20(1):184. https://doi.org/10.1186/s12886-020-01436-7
Romano JG, Schulz P, Kenkel S, Todd DP (2008) Visual field changes after a rehabilitation intervention: vision restoration therapy. J Neurol Sci 273(1–2):70–74. https://doi.org/10.1016/j.jns.2008.06.026
Ho RW, Huang HM, Ho JT (2015) The influence of pituitary adenoma size on vision and visual outcomes after trans-sphenoidal adenectomy: a report of 78 cases. J Korean Neurosurg Soc 57(1):23–31. https://doi.org/10.3340/jkns.2015.57.1.23
Lee J, Kim SW, Kim DW et al (2016) Predictive model for recovery of visual field after surgery of pituitary adenoma. J Neurooncol 130(1):155–164. https://doi.org/10.1007/s11060-016-2227-5
Wang MTM, King J, Symons RCA et al (2020) Prognostic utility of optical coherence tomography for long-term visual recovery following pituitary tumor surgery. Am J Ophthalmol 218:247–254. https://doi.org/10.1016/j.ajo.2020.06.004
Chung YS, Na M, Yoo J et al (2020) Optical coherent tomography predicts long-term visual outcome of pituitary adenoma surgery: new perspectives from a 5-year follow-up study. Neurosurgery 88(1):106–112. https://doi.org/10.1093/neuros/nyaa318
Anik I, Anik Y, Cabuk B et al (2018) Visual outcome of an endoscopic endonasal transsphenoidal approach in pituitary macroadenomas: quantitative assessment with diffusion tensor imaging early and long-term results. World Neurosurg 112:e691–e701. https://doi.org/10.1016/j.wneu.2018.01.134
Eriksson U, Alm A (2009) Repeatability in and interchangeability between the macular and the fast macular thickness map protocols: a study on normal eyes with Stratus optical coherence tomography. Acta Ophthalmol 87(7):725–730. https://doi.org/10.1111/j.1755-3768.2008.01345.x
Taghvaei M, Sadrehosseini SM, Ostadrahimi N, Sarraf P, Zeinalizadeh M (2019) Preoperative visual evoked potential in the prediction of visual outcome after pituitary macroadenomas surgery. Pituitary 22(4):397–404. https://doi.org/10.1007/s11102-019-00969-5
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762. https://doi.org/10.1038/nrclinonc.2017.141
Limkin EJ, Sun R, Dercle L et al (2017) Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncology 28(6):1191–1206. https://doi.org/10.1093/annonc/mdx034
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577. https://doi.org/10.1148/radiol.2015151169
Fleseriu M, Biller BMK, Freda PU et al (2021) A Pituitary Society update to acromegaly management guidelines. Pituitary 24(1):1–13. https://doi.org/10.1007/s11102-020-01091-7
Molitch ME (2017) Diagnosis and treatment of pituitary adenomas: a review. JAMA 317(5):516–524. https://doi.org/10.1001/jama.2016.19699
Avraham E, Azriel A, Melamed I et al (2020) The chiasmal compression index: an integrative assessment tool for visual disturbances in patients with pituitary macroadenomas. World Neurosurg 143:e44–e50. https://doi.org/10.1016/j.wneu.2020.06.086
Zamora C, Castillo M (2017) Sellar and Parasellar Imaging. Neurosurgery 80(1):17–38. https://doi.org/10.1093/neuros/nyw013
Yoneoka Y, Hatase T, Watanabe N et al (2015) Early morphological recovery of the optic chiasm is associated with excellent visual outcome in patients with compressive chiasmal syndrome caused by pituitary tumors. Neurol Res 37(1):1–8. https://doi.org/10.1179/1743132814y.0000000407
Peeken JC, Asadpour R, Specht K et al (2021) MRI-based delta-radiomics predicts pathologic complete response in high-grade soft-tissue sarcoma patients treated with neoadjuvant therapy. Radiother Oncol 164:73–82. https://doi.org/10.1016/j.radonc.2021.08.023
Jeon SH, Song C, Chie EK et al (2019) Delta-radiomics signature predicts treatment outcomes after preoperative chemoradiotherapy and surgery in rectal cancer. Radiat Oncol 14(1):43. https://doi.org/10.1186/s13014-019-1246-8
Crombé A, Périer C, Kind M et al (2019) T(2) -based MRI Delta-radiomics improve response prediction in soft-tissue sarcomas treated by neoadjuvant chemotherapy. J Magn Reson Imaging 50(2):497–510. https://doi.org/10.1002/jmri.26589
Sushentsev N, Rundo L, Blyuss O et al (2021) Comparative performance of MRI-derived PRECISE scores and delta-radiomics models for the prediction of prostate cancer progression in patients on active surveillance. Eur Radiol. https://doi.org/10.1007/s00330-021-08151-x
Yoo YJ, Hwang JM, Yang HK, Joo JD, Kim YH, Kim CY (2020) Prognostic value of macular ganglion cell layer thickness for visual outcome in parasellar tumors. J Neurol Sci 414:116823 https://doi.org/10.1016/j.jns.2020.116823
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Can Res 77(21):e104–e107. https://doi.org/10.1158/0008-5472.can-17-0339
Khanna O, Fathi Kazerooni A, Farrell CJ et al (2021) Machine learning using multiparametric magnetic resonance imaging radiomic feature analysis to predict Ki-67 in World Health Organization Grade I Meningiomas. Neurosurgery 89(5):928–936. https://doi.org/10.1093/neuros/nyab307
Van Calster B, Wynants L, Verbeek JFM et al (2018) Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol 74(6):796–804. https://doi.org/10.1016/j.eururo.2018.08.038
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Danesh-Meyer HV, Yoon JJ, Lawlor M, Savino PJ (2019) Visual loss and recovery in chiasmal compression. Prog Retin Eye Res 73:100765 https://doi.org/10.1016/j.preteyeres.2019.06.001
Metwali H, Giordano M, Kniese K, Fahlbusch R (2018) Prognostic significance of intraoperative change in the fractional anisotropy and the volume of the optic chiasma during resection of suprasellar tumors. J Neurosurg 128(5):1479–1485. https://doi.org/10.3171/2016.12.jns162741
Cellina M, Pirovano M, Ciocca M, Gibelli D, Floridi C, Oliva G (2021) Radiomic analysis of the optic nerve at the first episode of acute optic neuritis: an indicator of optic nerve pathology and a predictor of visual recovery? Radiol Med (Torino) 126(5):698–706. https://doi.org/10.1007/s11547-020-01318-4
Barzaghi LR, Medone M, Losa M, Bianchi S, Giovanelli M, Mortini P (2012) Prognostic factors of visual field improvement after trans-sphenoidal approach for pituitary macroadenomas: review of the literature and analysis by quantitative method. Neurosurg Rev 35(3):369–378; discussion 378–369 https://doi.org/10.1007/s10143-011-0365-y
Rivoal O, Brézin AP, Feldman-Billard S, Luton JP (2000) Goldmann perimetry in acromegaly: a survey of 307 cases from 1951 through 1996. Ophthalmology 107(5):991–997. https://doi.org/10.1016/s0161-6420(00)00060-9
Sun M, Zhang ZQ, Ma CY, Chen SH, Chen XJ (2017) Predictive factors of visual function recovery after pituitary adenoma resection: a literature review and Meta-analysis. Int J Ophthalmol 10(11):1742–1750. https://doi.org/10.18240/ijo.2017.11.17
Xia L, Wenhui J, Xiaowen Y et al (2022) Predictive value of macular ganglion cell-inner plexiform layer thickness in visual field defect of pituitary adenoma patients: a case-control study. Pituitary 25(4):667–672. https://doi.org/10.1007/s11102-022-01248-6
Meyer J, Diouf I, King J et al (2022) A comparison of macular ganglion cell and retinal nerve fibre layer optical coherence tomographic parameters as predictors of visual outcomes of surgery for pituitary tumours. Pituitary 25(4):563–572. https://doi.org/10.1007/s11102-022-01228-w
Lee GI, Son KY, Park KA, Kong DS, Oh SY (2021) Longitudinal changes in the retinal microstructures of eyes with chiasmal compression. Neurology 96(1):e131–e140. https://doi.org/10.1212/wnl.0000000000011087
Acknowledgements
The abstract of this study was presented as the electronic poster in European Congress of Radiology 2023.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chaoyue Chen.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No
Methodology
-
retrospective
-
diagnostic or prognostic study
-
performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Zheng, J., Huang, Z. et al. Predicting visual recovery in pituitary adenoma patients post-endoscopic endonasal transsphenoidal surgery: Harnessing delta-radiomics of the optic chiasm from MRI. Eur Radiol 33, 7482–7493 (2023). https://doi.org/10.1007/s00330-023-09963-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09963-9