Abstract
Objectives
Using contrast-enhanced computed tomography (CECT) and deep learning technology to develop a deep learning radiomics nomogram (DLRN) to preoperative predict risk status of patients with thymic epithelial tumors (TETs).
Methods
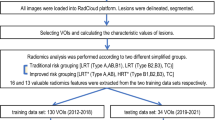
Between October 2008 and May 2020, 257 consecutive patients with surgically and pathologically confirmed TETs were enrolled from three medical centers. We extracted deep learning features from all lesions using a transformer-based convolutional neural network and created a deep learning signature (DLS) using selector operator regression and least absolute shrinkage. The predictive capability of a DLRN incorporating clinical characteristics, subjective CT findings and DLS was evaluated by the area under the curve (AUC) of a receiver operating characteristic curve.
Results
To construct a DLS, 25 deep learning features with non-zero coefficients were selected from 116 low-risk TETs (subtypes A, AB, and B1) and 141 high-risk TETs (subtypes B2, B3, and C). The combination of subjective CT features such as infiltration and DLS demonstrated the best performance in differentiating TETs risk status. The AUCs in the training, internal validation, external validation 1 and 2 cohorts were 0.959 (95% confidence interval [CI]: 0.924–0.993), 0.868 (95% CI: 0.765–0.970), 0.846 (95% CI: 0.750–0.942), and 0.846 (95% CI: 0.735–0.957), respectively. The DeLong test and decision in curve analysis revealed that the DLRN was the most predictive and clinically useful model.
Conclusions
The DLRN comprised of CECT-derived DLS and subjective CT findings showed a high performance in predicting risk status of patients with TETs.
Clinical relevance statement
Accurate risk status assessment of thymic epithelial tumors (TETs) may aid in determining whether preoperative neoadjuvant treatment is necessary. A deep learning radiomics nomogram incorporating enhancement CT-based deep learning features, clinical characteristics, and subjective CT findings has the potential to predict the histologic subtypes of TETs, which can facilitate decision-making and personalized therapy in clinical practice.
Key Points
• A non-invasive diagnostic method that can predict the pathological risk status may be useful for pretreatment stratification and prognostic evaluation in TET patients.
• DLRN demonstrated superior performance in differentiating the risk status of TETs when compared to the deep learning signature, radiomics signature, or clinical model.
• The DeLong test and decision in curve analysis revealed that the DLRN was the most predictive and clinically useful in differentiating the risk status of TETs.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CECT:
-
Contrast-enhanced computed tomography
- CI:
-
Confidence interval
- CNN:
-
Convolutional neural network
- CT:
-
Computed tomography
- DCA:
-
Decision curve analysis
- DL:
-
Deep learning
- DLRN:
-
Deep learning radiomics nomogram
- DLS:
-
Deep learning signature
- HE:
-
Hematoxylin-eosin
- IDI:
-
Integrated discrimination improvement
- LASSO:
-
Least absolute shrinkage and selection operator
- NPV:
-
Negative probability value
- NRI:
-
Net reclassification index
- OR:
-
Odds ratio
- PPV:
-
Positive probability value
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- RS:
-
Radiomics signature
- TETs:
-
Thymic epithelial tumors
References
de Jong WK, Blaauwgeers JLG, Schaapveld M et al (2008) Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 44(1):123–130. https://doi.org/10.1016/j.ejca.2007.11.004
Marx A, Chan JK, Coindre JM et al (2015) The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol 10(10):1383–1395. https://doi.org/10.1097/JTO.0000000000000654
Shintani Y, Funaki S, Ose N et al (2021) Surgical management of thymic epithelial tumors. Surg Today 51(3):331–339. https://doi.org/10.1007/s00595-020-02070-y
Molina TJ, Bluthgen MV, Chalabreysse L et al (2021) Impact of expert pathologic review of thymic epithelial tumours on diagnosis and management in a real-life setting: a RYTHMIC study. Eur J Cancer 143:158–167. https://doi.org/10.1016/j.ejca.2020.11.011
Süveg K, Putora PM, Joerger M et al (2021) Radiotherapy for thymic epithelial tumours: a review. Trasl Lung Cancer Res 10(4):2088–2100
Sato Y, Yanagawa M, Hata A et al (2018) Volumetric analysis of the thymic epithelial tumors: correlation of tumor volume with the WHO classification and Masaoka staging. J Thorac Dis 10(10):5822–5832
Hu YC, Wu L, Yan LF et al (2014) Predicting subtypes of thymic epithelial tumors using CT: new perspective based on a comprehensive analysis of 216 patients. Sci Rep 10(4):6984. https://doi.org/10.1038/srep06984
Moon JW, Lee KS, Shin MH et al (2015) Thymic epithelial tumors: prognostic determinants among clinical, histopathologic, and computed tomography findings. Ann Thorac Surg 99(2):462–470. https://doi.org/10.1016/j.athoracsur.2014.09.050
Choe J, Lee SM, Lim S et al (2017) Doubling time of thymic epithelial tumours on CT: correlation with histological subtype. Eur Radiol 27(10):4030–4036. https://doi.org/10.1007/s00330-017-4795-y
Han X, Gao W, Chen Y et al (2019) Relationship between computed tomography imaging features and clinical characteristics, Masaoka-Koga stages, and World Health Organization histological classifications of thymoma. Front Oncol 11(9):1041. https://doi.org/10.3389/fonc.2019.01041
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577. https://doi.org/10.1148/radiol.2015151169
Limkin EJ, Sun R, Dercle L et al (2017) Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol 28(6):1191–1206. https://doi.org/10.1093/annonc/mdx034
Yasaka K, Akai H, Nojima M et al (2017) Quantitative computed tomography texture analysis for estimating histological subtypes of thymic epithelial tumors. Eur J Radiol 92:84–92. https://doi.org/10.1016/j.ejrad.2017.04.017
Iannarelli A, Sacconi B, Tomei F et al (2018) Analysis of CT features and quantitative texture analysis in patients with thymic tumors: correlation with grading and staging. Radiol Med 123(5):345–350. https://doi.org/10.1007/s11547-017-0845-4
Kirienko M, Ninatti G, Cozzi L et al (2020) Computed tomography (CT)-derived radiomic features differentiate prevascular mediastinum masses as thymic neoplasms versus lymphomas. Radiol Med 125(10):951–960. https://doi.org/10.1007/s11547-020-01188-w
Zhao B, Tan Y, Tsai WY et al (2016) Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep 24(6):23428. https://doi.org/10.1038/srep23428
Mackin D, Fave X, Zhang L et al (2015) Measuring computed tomography scanner variability of radiomics features. Invest Radiol 50:757–765. https://doi.org/10.1097/RLI.0000000000000180
Zhovannik I, Bussink J, Traverso A et al (2019) Learning from scanners: bias reduction and feature correction in radiomics. Clin Transl Radiat Oncol 16(19):33–38. https://doi.org/10.1016/j.ctro.2019.07.003
Lotter W, Diab AR, Haslam B et al (2021) Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach. Nat Med 27(2):244–249. https://doi.org/10.1038/s41591-020-01174-9
Esteva A, Kuprel B, Novoa RA et al (2017) Dermatologist-level classification of skin cancer with deep neural networks. Nature 542(7639):115–118. https://doi.org/10.1038/nature21056
Song Z, Liu T, Shi L et al (2021) The deep learning model combining CT image and clinicopathological information for predicting ALK fusion status and response to ALK-TKI therapy in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 48(2):361–371. https://doi.org/10.1007/s00259-020-04986-6
Greenspan H, van Ginneken B, Summers RM (2016) Guest editorial deep learning in medical imaging: overview and future promise of an exciting new technique. IEEE Trans Med Imaging 35:1153–1159. https://doi.org/10.1109/TMI.2016.2553401
Lee SM, Seo JB, Yun J et al (2019) Deep learning applications in chest radiography and computed tomography current state of the art. J Thorac Imaging 34(2):75–85. https://doi.org/10.1097/RTI.0000000000000387
Castiglioni I, Rundo L, Codari M et al (2021) AI applications to medical images: from machine learning to deep learning. Phys Med 83:9–24. https://doi.org/10.1016/j.ejmp.2021.02.006
Travis WD, Brambilla E, Burke AP (2015) WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer 187–243.
Srinivas A, Lin TY, Parmar N et al (2021) Bottleneck transformers for visual recognition. https://doi.org/10.1109/CVPR46437.2021.01625
Wu G, Jochems A, Refaee T et al (2021) Structural and functional radiomics for lung cancer. Eur J Nucl Med Mol Imaging 48(12):3961–3974. https://doi.org/10.1007/s00259-021-05242-1
Nakajo M, Takeda A, Katsuki A et al (2022) The efficacy of 18F-FDG-PET-based radiomic and deep-learning features using a machine-learning approach to predict the pathological risk subtypes of thymic epithelial tumors. Br J Radiol 95(1134):20211050. https://doi.org/10.1259/bjr.20211050
Li Y, Wu FX, Ngom A (2018) A review on machine learning principles for multi-view biological data integration. Brief Bioinform 19(2):325–340. https://doi.org/10.1093/bib/bbw113
Sahiner B, Pezeshk A, Hadjiiski LM et al (2019) Deep learning in medical imaging and radiation therapy. Med Phys 46(1):1–36. https://doi.org/10.1002/mp.13264
Funding
This study has received funding from the National Natural Science Foundation of China (Grant Number: 62176104 and 81960324), the Natural Science Foundation of Guangxi Province (Grant Number: 2021GXNSFAA075037), and the Medical Research Foundation of Guangdong Province (Grant Number: A2021138).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Wansheng Long.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Bao Feng kindly provided statistical advice for this manuscript.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
Study subjects or cohorts have not been previously reported.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Feng, B., Xu, K. et al. Development and validation of a deep learning radiomics nomogram for preoperatively differentiating thymic epithelial tumor histologic subtypes. Eur Radiol 33, 6804–6816 (2023). https://doi.org/10.1007/s00330-023-09690-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09690-1