Abstract
Objectives
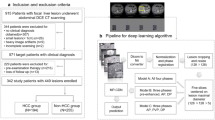
To determine whether a CT-based machine learning (ML) can differentiate benign renal tumors from renal cell carcinomas (RCCs) and improve radiologists’ diagnostic performance, and evaluate the impact of variable CT imaging phases, slices, tumor sizes, and region of interest (ROI) segmentation strategies.
Methods
Patients with pathologically proven RCCs and benign renal tumors from our institution between 2008 and 2020 were included as the training dataset for ML model development and internal validation (including 418 RCCs and 78 benign tumors), and patients from two independent institutions and a public database (TCIA) were included as the external dataset for individual testing (including 262 RCCs and 47 benign tumors). Features were extracted from three-phase CT images. CatBoost was used for feature selection and ML model establishment. The area under the receiver operating characteristic curve (AUC) was used to assess the performance of the ML model.
Results
The ML model based on 3D images performed better than that based on 2D images, with the highest AUC of 0.81 and accuracy (ACC) of 0.86. All three radiologists achieved better performance by referring to the classifier’s decision, with accuracies increasing from 0.82 to 0.87, 0.82 to 0.88, and 0.76 to 0.87. The ML model achieved higher negative predictive values (NPV, 0.82–0.99), and the radiologists achieved higher positive predictive values (PPV, 0.91–0.95).
Conclusions
A ML classifier based on whole-tumor three-phase CT images can be a useful and promising tool for differentiating RCCs from benign renal tumors. The ML model also perfectly complements radiologist interpretations.
Key Points
• A machine learning classifier based on CT images could be a reliable way to differentiate RCCs from benign renal tumors.
• The machine learning model perfectly complemented the radiologists’ interpretations.
• Subtle variances in ROI delineation had little effect on the performance of the ML classifier.





Similar content being viewed by others
Abbreviations
- ACC:
-
Accuracy
- AML:
-
Angiomyolipoma
- ASL:
-
Arterial spin labeling
- CECT:
-
Contrast-enhanced computed tomography
- CMP:
-
Corticomedullary phase
- DWI:
-
Diffusion-weighted imaging
- GLCM:
-
Gray-level cooccurrence matrix
- GLDM:
-
Gray-level dependence matrix
- GLRLM:
-
Gray-level run-length matrix
- GLSZM:
-
Gray-level size-zone matrix
- ML:
-
Machine learning
- NGTDM:
-
Neighborhood gray-tone difference matrix
- NP:
-
Nephrographic phase
- PCP:
-
Precontrast phase
- RCC:
-
Renal cell carcinoma
- ROI:
-
Region of interest
References
Vendrami CL, Villavicencio CP, Dejulio TJ et al (2017) Differentiation of solid renal tumors with multiparametric MR imaging. Radiographics 37(7):2026–2042. https://doi.org/10.1148/rg.2017170039
Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA (2004) The natural history of incidentally detected small renal masses. Cancer 100(4):738–745. https://doi.org/10.1002/cncr.20025
Gill IS, Aron M, Gervais DA, Jewett MAS (2010) Clinical practice. Small renal mass. N Engl J Med 362(7):624–634. https://doi.org/10.1056/NEJMcp0910041
Lane BR, Babineau D, Kattan MW et al (2007) A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol 178(2):429–434. https://doi.org/10.1016/j.juro.2007.03.106
Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC (2008) Renal mass biopsy--a renaissance? J Urol 179(1):20–27. https://doi.org/10.1016/j.juro.2007.08.124
de Leon AD, Kapur P, Pedrosa I (2019) Radiomics in kidney cancer: MR imaging. Magn Reson Imaging Clin N Am 27(1):1–13. https://doi.org/10.1016/j.mric.2018.08.005
Erdim C, Yardimci AH, Bektas CT et al (2020) Prediction of benign and malignant solid renal masses: machine learning-based CT texture analysis. Acad Radiol 27(10):1422–1429. https://doi.org/10.1016/j.acra.2019.12.015
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577. https://doi.org/10.1148/radiol.2015151169
Davnall F, Yip CSP, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3(6):573–589. https://doi.org/10.1007/s13244-012-0196-6
Suarez-Ibarrola R, Basulto-Martinez M, Heinze A, Gratzke C, Miernik A (2020) Radiomics applications in renal tumor assessment: a comprehensive review of the literature. Cancers (Basel) 12(6):1–25. https://doi.org/10.3390/cancers12061387
Tabibu S, Vinod PK, Jawahar CV (2019) Pan-renal cell carcinoma classification and survival prediction from histopathology images using deep learning. Sci Rep 9(1):1–9. https://doi.org/10.1038/s41598-019-46718-3
Sun XY, Feng QX, Xu X et al (2020) Radiologic-radiomic machine learning models for differentiation of benign and malignant solid renal masses: comparison with expert-level radiologists. AJR Am J Roentgenol 214(1):W44–W54. https://doi.org/10.2214/AJR.19.21617
Cui E, Li Z, Ma C et al (2020) Predicting the ISUP grade of clear cell renal cell carcinoma with multiparametric MR and multiphase CT radiomics. Eur Radiol 30(5):2912–2921. https://doi.org/10.1007/s00330-019-06601-1
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Cui EM, Lin F, Li Q et al (2019) Differentiation of renal angiomyolipoma without visible fat from renal cell carcinoma by machine learning based on whole-tumor computed tomography texture features. Acta Radiol 60(11):1543–1552. https://doi.org/10.1177/0284185119830282
Chen F, Gulati M, Hwang D et al (2017) Voxel-based whole-lesion enhancement parameters: a study of its clinical value in differentiating clear cell renal cell carcinoma from renal oncocytoma. Abdom Radiol (NY) 42(2):552–560. https://doi.org/10.1007/s00261-016-0891-8
Mazzei FG, Mazzei MA, Cioffi Squitieri N et al (2014) CT perfusion in the characterisation of renal lesions: an added value to multiphasic CT. Biomed Res Int 2014:135013. https://doi.org/10.1155/2014/135013
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. Stud Media Commun SMC 3(6):610–621. 10.1109/TSMC.1973.4309314.
Varghese BA, Chen F, Hwang DH (2018) Differentiation of predominantly solid enhancing lipid-poor renal cell masses by use of contrast-enhanced CT: evaluating the role of texture in tumor subtyping. AJR Am J Roentgenol 211(6):W288–W296. https://doi.org/10.2214/AJR.18.19551
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77(21):e104–e107. https://doi.org/10.1158/0008-5472.CAN-17-0339
Prokhorenkova L, Gusev G, Vorobev A, Dorogush AV, Gulin A (2018) Catboost: unbiased boosting with categorical features. Adv Neural Inf Process Syst 2018-Decem:6638–6648. https://doi.org/10.48550/arXiv.1706.09516
Dorogush AV, Ershov V, Gulin A (2018) CatBoost: gradient boosting with categorical features support. 1–7. https://doi.org/10.48550/arXiv.1810.11363
Dyer R, DiSantis DJ, McClennan BL (2008) Simplified imaging approach for evaluation of the solid renal mass in adults. Radiology 247(2):331–343. https://doi.org/10.1148/radiol.2472061846
Leveridge MJ, Bostrom PJ, Koulouris G, Finelli A, Lawrentschuk N (2010) Imaging renal cell carcinoma with ultrasonography, CT and MRI. Nat Rev Urol 7(6):311–325. https://doi.org/10.1038/nrurol.2010.63
Kutikov A, Smaldone MC, Egleston BL et al (2011) Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol 60(2):241–248. https://doi.org/10.1016/j.eururo.2011.03.029
Hunter JD (2007) Matplotlib: a 2D graphics environment. Comput Sci Eng 9(3):90–95. https://doi.org/10.1109/MCSE.2007.55
Kunapuli G, Varghese BA, Ganapathy P et al (2018) A decision-support tool for renal mass classification. J Digit Imaging 31(6):929–939. https://doi.org/10.1007/s10278-018-0100-0
Xi IL, Zhao Y, Wang R et al (2020) Deep learning to distinguish benign from malignant renal lesions based on routine MR imaging. Clin Cancer Res 26(8):1944–1952. https://doi.org/10.1158/1078-0432.CCR-19-0374
Tanaka T, Huang Y, Marukawa Y et al (2020) Differentiation of small (≤ 4 cm) renal masses on multiphase contrast-enhanced CT by deep learning. AJR Am J Roentgenol 214(3):605–612. https://doi.org/10.2214/AJR.19.22074
Acknowledgements
I would like to express my gratitude to Professor Wansheng Long, my supervisor, for his constant encouragement, guidance, and enlightening instructions, which contributed to the completion of my paper. I would also like to acknowledge my indebtedness to Enming Cui and Fan Lin, without whose valuable resources and support this paper could not have appeared in its final form. I am also grateful to all the other teachers and classmates who have given me generous support and helpful advice in the course of shaping my paper. Last but not least, my thanks go to my beloved families, for their loving considerations and great confidence in me all through these years.
Funding
This study has received funding from Guangdong Basic and Applied Basic Research Foundation (Grant Number: 2021A1515220080) and the Opening Research Fund of Guangzhou Key Laboratory of Molecular and Functional Imaging for Clinical Translation (Grant Number: 201905010003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Wansheng Long.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, T., Guan, J., Feng, B. et al. Distinguishing common renal cell carcinomas from benign renal tumors based on machine learning: comparing various CT imaging phases, slices, tumor sizes, and ROI segmentation strategies. Eur Radiol 33, 4323–4332 (2023). https://doi.org/10.1007/s00330-022-09384-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09384-0