Abstract
Objectives
To evaluate the preoperative diagnostic value of contrast-enhanced lymphatic ultrasound (CEUS) for the sentinel lymph node (SLN) status in early breast cancer.
Materials and methods
We prospectively recruited 102 consecutive patients with clinically node-negative early breast cancer from July 2021 to October 2021. All patients underwent conventional US and percutaneous CEUS examinations. The CEUS of SLNs were classified into four enhancement patterns: homogeneous (I), featured inhomogeneous (II), focal defect (III), and no enhancement (IV). The diagnostic performance of conventional US and CEUS for SLN metastasis was assessed by receiver operating characteristic (ROC) curves and decision curves.
Results
A total of 78 women were enrolled in this study, including 55, 18, and 5 patients with negative axilla, 1–2, and ≥ 3 metastastic SLNs pathologically, respectively. The identification rate of SLNs by CEUS was 100%. Patterns I and II can select 91.7% (44/48) of patients with disease-free axilla, while patterns III and IV had higher percentages of metastasis (65.2%, p < 0.001 and 57.1%, p < 0.002, respectively). For the SLN metastatic burden, 100% (48/48) of patients with pattern I/II had ≤ 2 metastatic SLNs. Compared with conventional US, the CEUS enhancement patterns showed significant improvement in diagnosing metastatic SLNs (0.813 vs 0.601, p < 0.001). CEUS had greater clinical benefits and correctly reclassified 48% of metastatic SLNs (p < 0.001) without sacrificing the classification accuracy of negative SLNs (p = 0.25), and could improve prediction accuracy by 0.42 (p < 0.001).
Conclusions
CEUS demonstrated better diagnostic performance and greater clinical benefits than conventional US for the preoperative diagnosis of SLNs, showing its potential to select candidates for precluding axillary surgery in early breast cancer.
Key Points
• The homogeneous and featured inhomogeneous enhancement of SLNs are highly suggestive of negative LNs, while focal defect (p < 0.001) and no enhancement (p < 0.002) patterns had higher percentages of metastasis.
• The proportion of SLNs with highly suspicious signs on conventional US increases as the type of enhancement pattern increases (no suspicious signs in pattern I/II, 34.8% in pattern III, and 85.7% in pattern IV).
• Compared with conventional US, CEUS improved the area under the receiver operating characteristic curve (0.813 vs. 0.601, p < 0.001) and had greater clinical benefits (IDI = 0.42, p < 0.001) for the diagnosis of axillary metastasis.
Similar content being viewed by others
Introduction
Sentinel lymph node biopsy (SLNB) has been the standard procedure for axillary lymph node (ALN) staging in breast cancer. According to American College of Surgeons Oncology Group (ACOSOG) Z0011 data, ALN dissection (ALND) can be replaced by sentinel lymph node biopsy (SLNB) for clinical T1-2 N0 breast cancer patients with 1–2 metastatic SLNs [1, 2]. However, for patients with early breast cancer and nonpalpable axillary LNs, approximately 29–45% of nodes are pathologically positive, with a very small percentage of patients having ≥ 3 positive nodes [3,4,5]. With the application of high-quality mammographic screening, SLN positivity rates were reported to be below 20% [6], which means that the majority of patients without ALN metastasis underwent a wider axillary surgery extent. Preoperative imaging assessment of SLN will help to stratify patients with early breast cancer and provide information for selecting the axillary surgery approach.
Among the various preoperative imaging modalities, ultrasound (US) is the primary method to evaluate the axilla in breast cancer patients. Conventional US demonstrated relevant variability in sensitivity (26–87%) and specificity (55–98%) in the evaluation of nonpalpable LNs [7]. However, conventional US, computed tomography (CT), and magnetic resonance imaging (MRI) fail to localize the SLNs [8]. With the development of lymphatic contrast-enhanced (CE) US, SLNs can be identified by US contrast agents through the lymphatic route with percutaneous injection [9, 10]. The SLNs identified by CEUS are consistent with the intraoperative SLNs identified by blue dye localization [11, 12]. The technique of percutaneous CEUS, as a simple and noninvasive method, expanded a new field for the preoperative assessment of SLNs.
The CEUS patterns of SLNs include homogeneous, inhomogeneous, and no enhancement patterns. SLNs with homogeneous enhancement tend to be negative LNs, and inhomogeneous or no enhancement patterns are highly suggestive of metastatic LNs [12,13,14]. However, the enhancement pattern can occur in both benign and malignant SLNs with a great overlap [15], and the detailed classification of SLN enhancement patterns and their relationship with the final SLN status has not yet been well established. If the further classification of CEUS enhancement patterns can improve the preoperative diagnostic accuracy for SLNs in clinical T1-2N0 breast cancer, it could benefit patients with an alternative to SLNB in a very low-risk population.
Therefore, the purpose of this study was to investigate the ability of CEUS enhancement patterns to accurately diagnose SLN in early breast cancer and compare the diagnostic performance with that of conventional US.
Materials and methods
This prospective study was approved by the institutional review board and local ethics committee. All participants signed informed consent forms.
Study design and population
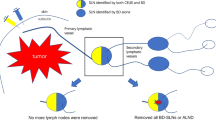
Consecutive patients with pathologically confirmed clinical T1-2 breast cancer with negative axillary lymph nodes on palpation were recruited for this study from July 2021 to October 2021. All patients had no US contrast agent contraindication and no medical history of ipsilateral axillary surgery or radiotherapy. The exclusion criteria were as follows: (i) neoadjuvant therapy before US examination and (ii) no axillary surgery planned. The flow chart of this study is shown in Fig. 1.
Conventional US examination
A high-frequency linear array transducer 12-5 MHz was used in this study. Conventional US was performed on the breast and axilla. Ultrasound images of suspected axillary lymph nodes were stored in all cases. By conventional axillary US, any axillary lymph node with suspicious signs that met two or more of the following criteria was considered metastatic: ratio of the long diameter to the short diameter (L/S) < 2, unclear margin or an irregular shape, compression or absence of the fatty hilum, cortical thickness ≥ 3 mm or asymmetry, merged lymph nodes and rich blood flow signal [16, 17].
An experienced radiologist (Z.Q.L.) performed the axilla US and made the diagnosis onsite. Those conventional US images were independently analysed by another radiologist (N.Z.H.). Both had completed over 5000 breast US examinations. They were blinded to the final pathological results. If there were differences of opinion, they reached an agreement through discussion.
Contrast-enhanced ultrasound (CEUS) image acquisition
The contrast agent (SonoVue, Bracco Imaging or Sonazoid, GE Healthcare AS) was mixed with 2 mL of sterile saline. Approximately 0.5 mL of contrast agent was intradermally injected into the peri-areolar area at the 3, 6, 9, and 12 o’clock positions, followed by a 10-s massage on each injection site. With the mode of contrast pulse sequences (CPS), the low mechanical index (MI) value was set to 0.06–0.10. By following the enhanced lymphatic channel on the CPS, the first enhanced lymph node(s) can be located. The live dual images mode was used to confirm the presence of an architecturally defined SLN. For every patient, up to 2 mL (4 injections) of the agent could be injected if the SLN was not seen. The enhanced lymphatic duct and SLNs on the skin surface were marked under the guidance of CEUS.
Based on previous experiences, only one SLN could be identified in most cases, and sometimes the second or even third node could be enhanced via the efferent lymphatic vessels of the first node. Furthermore, several lymph nodes could be detected simultaneously through parallel lymphatic vessels in some cases. If more than one SLN was present, the SLN with the highest category enhancement pattern or the first SLN was regarded as the index SLN.
CEUS patterns of SLNs
Referring to previous studies [12,13,14] and our experiences, the enhancement patterns of SLNs included four patterns.
Pattern I showed homogeneous enhancement, and the entire lymph node showed bright, homogeneous enhancement (Fig. 2a).
The four types of CEUS enhancement patterns of SLNs. With the live-dual mode, the enhancement pattern (left) and the greyscale (right) images are shown in figures. A Pattern I, homogeneous enhancement. The entire lymph node showed bright, homogeneous enhancement. B Pattern II, cribriform enhancement. The SLN had a low enhancement with an even distribution inside the node resembling a sieve mesh. C Pattern II, half-moon enhancement, a semilunar, homogeneous enhancement with a centred afferent lymph vessel or enhancement of the cortex with a contrast agent. D Pattern II, ring enhancement, a regular, uniform, bright ring enhancement at the periphery of the node. E Pattern III, focal defect enhancement. The uneven distribution of the contrast agent with filling defect areas (*) of the SLN is seen correlating to the focal eccentric cortical thickening (arrow) on grayscale US. F Pattern IV, no enhancement. The SLN showed no enhancement in the whole region (*) and focal eccentric cortical thickening (arrow) on grayscale US. US, ultrasound; CEUS, contrast-enhanced lymphatic US; SLN, sentinel lymph node
Pattern II showed inhomogeneous enhancement, including cribriform (Fig. 2b), half-moon (Fig. 2c), and ring (Fig. 2d) enhancement. Pattern II was diagnosed only if the SLN identified by CEUS was not suspicious on conventional ultrasound. Cribriform enhancement is a low enhancement with an even distribution inside the node resembling a sieve mesh. Ring enhancement is a regular, uniform, bright ring enhancement at the periphery of the node. Half-moon enhancement is a semilunar, homogeneous enhancement with a centred afferent lymph vessel or enhancement of the cortex with a contrast agent.
Pattern III showed focal defect enhancement (Fig. 2e). The enhancement of the lymph nodes was inhomogeneous, with a focal nonenhancement area beneath the node membrane.
Pattern IV showed no enhancement (Fig. 2f). No contrast agent entered the node by following the end of the enhanced afferent lymphatic vessels.
The CEUS enhancement images of SLNs were collected by an experienced radiologist (Z.Q.L.), and the diagnosis was made on-site. The patterns of SLNs were independently reviewed and analysed by another ultrasound physician (N.Z.H.) based on re-readings of the cine-loops. They were blinded to the conventional US outcomes and final pathological results. If there was a disagreement, they reached a consensus through discussion.
Surgical management of SLNs
During the operation, blue dye and indocyanine green (ICG) were intradermally injected into the periareolar tissue to identify SLNs. All blue-stained and ICG-stained lymph nodes were resected and subjected to standard histological analysis. SLNs with a metastatic tumour area of 0.2–2 mm were defined as micrometastatic LNs, and those with a metastatic tumour area of > 2 mm were defined as macrometastatic LNs [18]. Both micrometastasis and macrometastasis were defined as metastatic SLNs. The final evaluation of SLN was negative (SLN−), 1–2 metastases (SLN+ (1–2)), and ≥ 3 metastatic SLNs (SLN+ (≥ 3)). For each axilla, the enhancement pattern of the designated SLN corresponds to the SLN pathological status.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD) or the median (interquartile range), and categorical variables are expressed as numbers and percentages (%). The interreader agreement for the CEUS patterns was evaluated by Cohen’s kappa coefficient. For the comparison of conventional US and CEUS, the DeLong test was used to compare the AUCs of different models [19]. The diagnostic value of conventional US and CEUS was evaluated by calculating the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC) values were used to assess the discriminative ability of the diagnostic models. Decision curve analysis (DCA) was conducted to determine the clinical usefulness of different US methods by quantifying the net benefits at different threshold probabilities [20]. The integrated discrimination improvement (IDI) and net reclassification index (NRI) were calculated to reveal the predictive accuracy improvement of CEUS. The greater the curve deviated from the baseline, the greater the benefit was. All of the statistical analyses were performed using R (http://www.R-project. org), Empower Stats software (X&Y Solutions), and MedCalc (version 17.9.7).
Results
Baseline characteristics
Between July 2021 and October 2021, 102 consecutive women with clinical T1-2 breast cancer and negative axillary on palpation were considered for inclusion in the study.
Twenty-four women were excluded: 2 women with neoadjuvant therapy before CEUS and 22 without an axillary surgery plan. Finally, a total of 78 patients underwent CEUS and were included for analysis. The median size of breast cancer tumours was 1.8 cm, including 59 (75.6%) invasive ductal carcinomas, 10 (12.8%) invasive lobular carcinomas, and 9 (11.5%) other types of invasive carcinomas. The rate of SLN identification by CEUS was 100% (78/78). Negative SLNs, 1–2 metastatic SLNs, and ≥ 3 metastatic SLNs were observed in 55 (70.5%), 18 (23.1%), and 5 (6.4%) patients, respectively. The clinical baseline characteristics of the enrolled patients are shown in Table 1.
Enhancement patterns of SLNs
There were 19, 29, 23, and 7 SLNs showing enhancement patterns I, II, III, and IV, respectively. All of the SLNs with homogeneous pattern I were disease-free axillary SLNs. Pattern II, featured inhomogeneous enhancement, had 11, 10, and 8 SLNs in cribriform, ring, and half-moon enhancement, respectively. A total of 13.8% (4/29) of false-negative cases of SLNs were found in pattern II, including 2 cases of cribriform enhancement and 2 cases of ring enhancement patterns. All of the SLNs with half-moon enhancement were negative cases and appear to be a pattern predicting disease-free cases. SLNs with focal defects (pattern III) and no enhancement (pattern IV) had higher percentages of metastasis (65.2%, p < 0.001 and 57.1%, p < 0.002, respectively). Regarding the diagnosis of SLN metastatic burden, pattern II had a low SLN metastatic burden (≤ 2 metastatic lymph nodes). A total of 17.4% of pattern III patients and 14.3% of pattern IV patients harboured a high tumour burden (≥ 3 metastatic lymph nodes).
In this study, micrometastasis involving only one lymph node was found in 5 cases. Among them, 3 cases presented as pattern II, including 1 cribriform and 2 half-moon enhancement patterns. And the other 2 cases had inhomogeneous enhancement (pattern III). The characteristics of the enhancement patterns and the SLN status are shown in Table 2.
Correlation between SLN morphological characteristics and CEUS characteristics
Both homogeneous enhancement (pattern I) and featured inhomogeneous enhancement (pattern II) showed normal morphologic lymph nodes with thin cortical thickness and regular lymphatic hilum structure on conventional ultrasound (Fig. 1a-d). In contrast, there were 34.8% (8/23) of SLNs with high suspicion of malignancy in pattern III and 85.7% (6/7) in pattern IV. The SLNs presented at least one of the following suspicious features: an unclear margin or an irregular shape, compression or even the absence of the fatty hilum, cortical thickness ≥ 3 mm or asymmetry, and merged lymph nodes (Fig. 1f).
Comparison of the diagnostic value of conventional US and CEUS
The diagnostic values of different US characteristics are presented in Table 3. The CEUS patterns I and II were defined as negative; otherwise, they were considered positive. The accuracies of conventional US and CEUS were 70.5% and 80.8%, respectively. The NPV (91.7% vs. 75.8%) and sensitivity (82.6% vs. 34.8%) were largely increased by CEUS. For the diagnosis of positive or negative SLNs, the DeLong test for the area under the ROC curve demonstrated significant differences between CEUS (0.813[0.718,0.909]) and conventional US (0.601[0.491, 0.711]) (p < 0.001) (Fig. 3a).
The CEUS and conventional US for diagnosis of metastatic SLNs. a The receiver operating characteristic (ROC) curves of conventional US (blue line) and CEUS enhancement pattern (red line). b Decision curves of conventional US (blue line) and CEUS enhancement pattern (red line) for metastatic SLNs. US, ultrasound; CEUS, contrast-enhanced lymphatic US; SLN, sentinel lymph node; ROC, the receiver operating characteristic
The clinical application value of the two US methods was analysed via DCA (Fig. 3b). Compared with conventional US, CEUS had a higher overall net benefit at any given threshold probability. The NRI and IDI were measured to quantify the prediction accuracy of the different US methods (Table 4). The NRI+ indicated that CEUS could correctly reclassify 48% of metastatic SLN cases (p < 0.001). For negative SLN cases, CEUS and conventional US showed no significant difference in the classification accuracy of patients (NRI−: −0.05, p = 0.25). However, SLNs may be identified and localized using CEUS, which plays a more precise preoperative staging role than conventional US. IDI indicated that CEUS could improve prediction accuracy by 0.42 (p < 0.001) compared to conventional US.
Interobserver agreement of CEUS patterns
For CEUS patterns, two readers had the same classification in 63/78 (80.8%) SLNs, including homogeneous (16/19), featured heterogeneous (25/29), focal defect (18/23), and no enhancement (4/7) patterns. In the remaining 15 SLNs, the patterns were discussed, and an agreement was reached by the two doctors. Cohen’s kappa was 0.73, indicating good interreader agreement. For the conventional US, the two readers had the same classification in 61 negative and 13 positive cases. In the remaining 4 SLNs, the patterns were discussed, and an agreement was reached by the two doctors. The kappa value was 0.84, indicating excellent interreader agreement.
Discussion
The technique of percutaneous CEUS can identify SLNs and expand a new field for the preoperative assessment of SLNs. In this study, CEUS was applied to cT1-2N0 breast cancer patients, and the inhomogeneous enhancement pattern was further divided into featured inhomogeneous and focal defect enhancement. SLNs with homogeneous (pattern I) and featuring inhomogeneous enhancement (pattern II) are highly suggestive of negative SLNs, which can safely select 91.7% (44/48) women with disease-free axilla. The CEUS enhancement patterns (AUC: 0.813) showed significant improvement in diagnosing metastatic SLNs compared with conventional US (AUC: 0.601) and showed higher clinical usefulness at any given threshold probability. CEUS is a promising preoperative imaging method to stratify early-stage breast cancer patients. It might have the potential to select candidates who can be exempted from SLNB, which is beneficial for reducing surgical costs and complications.
For the evaluation of SLNs, many studies have confirmed that intradermal CEUS is as good as the accepted techniques for SLN detection [9, 10]. Then, further CEUS-guided SLN core biopsy showed a higher sensitivity for depicting metastases within the SLN. However, SLNB is still recommended since metastatic foci found by CEUS biopsy finally proved to be a low tumour burden [21,22,23]. Whether preoperative CEUS enhancement patterns could be feasible for lymph node status evaluation has attracted attention recently. Based on the initial three enhancement patterns, CEUS showed higher sensitivity (82–100%) but lower specificity (49–95%) [14]. Previous studies have shown that cortical filling or complete annular was a characteristic pattern for noninvolved lymph nodes, thus helping to improve specificity [24, 25]. This study further defined new featured patterns and explored their correlation with the final pathology. Pattern II is highly suggestive of a negative SLN, which might further preoperatively identify patients omitting SLNB. In this study, all patients harbouring ≥ 3 metastatic lymph nodes were classified as pattern III or IV, which implies that they might benefit from the neoadjuvant downgrading therapy or direct ALND. Further studies are warranted to verify the value of CEUS for selecting candidates for direct ALND.
The nonenhanced area in positive SLNs was thought to be associated with the accumulation of metastatic tumour cells obstructing or destroying small lymphatic vessels, from the lymphatic sinuses surrounding the lymphatic input vessels to medullary sinuses, and the entire LN structure [26, 27]. The contrast agent simulated the route of lymphatic drainage, and the degree of SLN filling defects increased as the number of invading tumour cells increased. Therefore, as the enhancement pattern grade increased, the percentage of SLNs with suspicious signs of malignancy was higher. However, the contrast agent in lymph flow is also influenced by various mechanical forces and solid stresses, which cause heterogeneous enhancement in negative SLNs [27, 28]. It is worth noting that the featured inhomogeneous pattern II shows uniform distribution of enhanced areas adjacent to afferent lymphatic vessels, and the relatively nonenhanced area is located in the medullary sinuses or around the efferent channels. Vigilance should be exercised for SLNs with a focal nonenhancement area beneath the node membrane to prevent false-negative cases. In summary, the enhancement distribution inside the node and the relative position of the input lymphatic vessels should be the CEUS assessment priorities.
The clinical significance of micrometastasis and macrometastasis of the SLN is still somewhat controversial [29,30,31,32]. Although several studies confirmed that the prognostic impact of micrometastases was similar to that of macrometastasis and significantly worse than disease-free axilla [33], ALND is not recommended for patients with only micrometastases of SLNs depicted according to the NCCN guidelines [18]. In this study, 4/29 patients with featured inhomogeneous enhancement pattern II showed metastatic SLNs, including 2 patients with micrometastasis and 2 patients with macrometastasis in 1 or 2 SLNs, which implies that all patients with pattern II are eligible for omitting ALND according to the NCCN guidelines. Pattern II can be used to safely screen patients with early breast cancer who can omit ALND before surgery and help optimize management.
The study was performed during the COVID-19 pandemic year 2021. Axillary lymphadenopathy was frequently observed after the introduction of COVID-19 vaccination, which is a confounding factor in the evaluation of the ipsilateral axilla in breast cancer patients [34]. Current evidence demonstrated that the US morphologic characteristics of lymphadenopathy post-COVID-19 vaccination usually tend to be diffuse cortical thickening, which is different from suspicious metastatic LN, such as focal cortical thickening and hilum effacement [35, 36]. Using the subcapsular injection of the ultrasonic contrast agent, Liu et al reported that reactive hyperplasia exhibited diffuse homogeneous or a brush-like enhancement from the subcapsular sinus to the centre without lymphatic tract distortion, while metastatic LN had lymphatic tract interruptions and contrast agent local accumulation [37]. This difference may be due to the lymphocytes’ proliferation uniformly without destroying the reticuloendothelial structure in reactive LN compared to the space-occupying effect of metastatic tumour cells. It implied that the percutaneous CEUS perfusion pattern might provide more information on the evaluation of axillary lymph nodes in the setting of the COVID-19 vaccine.
There are some limitations to this study. The main limitation of the study is the low number of included cases. Second, only the SLN with the highest enhancement pattern or first enhanced SLN was analysed in this study, since it has a one-to-one relationship with the axillary pathological outcome. Approximately 1–3 SLNs were identified in some cases. Further analysis for all enhanced SLNs is useful for the diagnosis of axillary tumours.
Conclusion
This study further classified the enhancement patterns of SLNs and found that homogeneous and featured inhomogeneous enhancement patterns are highly suggestive of negative SLNs. Compared with conventional US, CEUS showed higher diagnostic efficiency and greater clinical usefulness for disease-free axillary cancer. CEUS, as a more accurate method, is currently the best preoperative method for screening candidates with early breast cancer to omit SLNB. More cases are needed to validate the conclusion of this study.
Abbreviations
- ALN:
-
Axillary lymph node
- ALND:
-
Axillary lymph node dissection
- AUC:
-
Area under the receiver operating characteristic curve
- CEUS:
-
Contrast-enhanced ultrasound
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- ROC:
-
Receiver operating characteristic
- SLN:
-
Sentinel lymph node
- SLNB:
-
Sentinel lymph node biopsy
References
Giuliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6):569–575
Giuliano AE, Ballman KV, McCall L et al (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 318(10):918–926
Voogd AC, Coebergh JW, Repelaer van Driel OJ et al (2000) The risk of nodal metastases in breast cancer patients with clinically negative lymph nodes: a population-based analysis. Breast Cancer Res Treat 62(1):63–69
Giuliano AE, Haigh PI, Brennan MB et al (2000) Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 18(13):2553–2559
Krag DN, Anderson SJ, Julian TB et al (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11:927–933
Reimer T, Engel J, Schmidt M, Offersen BV, Smidt ML, Gentilini OD (2018) Is axillary sentinel lymph node biopsy required in patients who undergo primary breast surgery? Breast Care (Basel) 13:324–330
Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J (2006) Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol 186(5):1342–1348
Marino MA, Avendano D, Zapata P, Riedl CC, Pinker K (2020) Lymph Node Imaging in Patients with Primary Breast Cancer: Concurrent Diagnostic Tools. Oncologist 25(2):e231–e242
Goldberg BB, Merton DA, Liu JB et al (2004) Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 230(3):727–734
Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P (2009) Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 96(11):1295–1299
Esfehani MH, Yazdankhah-Kenari A, Omranipour R et al (2015) Validation of contrast enhanced ultrasound technique to wire localization of sentinel lymph node in patients with early breast cancer. Indian J Surg Oncol 6(4):370–373
Xie F, Zhang D, Cheng L et al (2015) Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J Surg Oncol 19(13):319
Li J, Lu M, Cheng X et al (2019) How pre-operative sentinel lymph node contrast-enhanced ultrasound helps intra-operative sentinel lymph node biopsy in breast cancer: initial experience. Ultrasound Med Biol 45(8):1865–1873
Zhao J, Zhang J, Zhu QL et al (2018) The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: a prospective study. Eur Radiol 28(4):1654–1661
Niu Z, Xiao M, Ma L et al (2022) The value of contrast-enhanced ultrasound enhancement patterns for the diagnosis of sentinel lymph node status in breast cancer: systematic review and meta-analysis. Quant Imaging Med Surg 12(2):936–948
Zhang YN, Wang CJ, Xu Y et al (2015) Sensitivity, specificity and accuracy of ultrasound in diagnosis of breast cancer metastasis to the axillary lymph nodes in Chinese patients. Ultrasound Med Biol 41(7):1835–1841
Chang JM, Shin HJ, Choi JS et al (2021) Imaging protocol and criteria for evaluation of axillary lymph nodes in the NAUTILUS trial. J Breast Cancer 24(6):554–560
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Published 2019.
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing areas under two or more correlated receiver operating characteristics curves: a nonparamentric approach. Biometrics 44(3):837–845
Vickers AJ, Cronin AM, Elkin EB, Gonen M (2008) Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak 8:53
Hieken T, Trull B, Boughey J et al (2013) Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery 154(4):831–840
Caudle AS, Kuerer HM, Le-Petross HT et al (2014) Predicting the extent of nodal disease in early-stage breast cancer. Ann Surg Oncol 21:3440–3447
Nielsen Moody A, Cox K, Haigh I, Chen Y, Sharma N (2020) Does contrast enhanced ultrasound (CEUS) of normal/benign axillary lymph nodes in patients with breast cancer identify significant axillary nodal burden? Eur J Radiol 132:109311
Hao Y, Sun Y, Lei Y, Zhao H, Cui L (2021) Percutaneous Sonazoid-enhanced ultrasonography combined with in vitro verification for detection and characterization of sentinel lymph nodes in early breast cancer. Eur Radiol 31(8):5894–5901
Jin L, Wang R, Zhuang L et al (2022) Evaluation of whole axillary status with lymphatic contrast-enhanced ultrasound in patients with breast cancer. Eur Radiol 32(1):630–638
Macdonald AJ, Arkill KP, Tabor GR, McHale NG, Winlove CP (2008) Modeling flow in collecting lymphatic vessels: one-dimensional flow through a series of contractile elements. Am J Physiol Heart Circ Physiol 295(1):H305–H313
Breslin JW (2014) Mechanical forces and lymphatic transport. Microvasc Res 96:46–54
Jones D, Wang Z, Chen IX et al (2021) Solid stress impairs lymphocyte infiltration into lymph-node metastases. Nat Biomed Eng 5(12):1426–1436
Gobardhan PD, Elias SG, Madsen EV et al (2009) Prognostic value of micrometastases in sentinel lymph nodes of patients with breast carcinoma: a cohort study. Ann Oncol 20(1):41–48
Houvenaeghel G, Classe JM, Garbay JR et al (2016) Survival impact and predictive factors of axillary recurrence after sentinel biopsy. Eur J Cancer 58:73–82
van Roozendaal LM, Schipper RJ, Van de Vijver KK et al (2014) The impact of the pathological lymph node status on adjuvant systemic treatment recommendations in clinically node negative breast cancer patients. Breast Cancer Res Treat 143(3):469–476
Houvenaeghel G, Classe JM, Garbay JR et al (2014) Prognostic value of isolated tumor cells and micrometastases of lymph nodes in early-stage breast cancer: a French sentinel node multicenter cohort study. Breast 23(5):561–566
Andersson Y, Bergkvist L, Frisell J, de Boniface J (2018) Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Res Treat 171(2):359–369
Chang JM, Ha SM (2022) Regional lymphadenopathy following COVID-19 vaccination in patients with or suspicious of breast cancer: a quick summary of current key facts and recommendations. Korean J Radiol. https://doi.org/10.3348/kjr.2022.0292
van Nijnatten TJA, Jochelson MS, Lobbes MBI (2022) Axillary lymph node characteristics in breast cancer patients versus post-COVID-19 vaccination: Overview of current evidence per imaging modality. Eur J Radiol 152:110334
Mehta N, Sales RM, Babagbemi K et al (2021) Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging 75:12–15
Liu SR, Liu C, Jing HM et al (2020) Subcapsular injection of ultrasonic contrast agent distinguishes between benign and malignant lymph node lesions exhibiting homogeneous enhancement in intravenous contrast-enhanced ultrasound images. Ultrasound Med Biol 46(3):582–588
Funding
This study has received funding by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS) (2020-I2M-C&T-B-033) and National Natural Sciences Foundation of China (82171967).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Qingli Zhu and Yuxin Jiang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Zihan Niu and Qingli Zhu did statistical analysis.
Informed consent
All participants signed informed consent forms.
Ethical approval
Institutional Review Board approval was obtained by Peking Union Medical College Hospital (NO. ZS-2291).
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, Z., Gao, Y., Xiao, M. et al. Contrast-enhanced lymphatic US can improve the preoperative diagnostic performance for sentinel lymph nodes in early breast cancer. Eur Radiol 33, 1593–1602 (2023). https://doi.org/10.1007/s00330-022-09139-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09139-x