Abstract
Objectives
To evaluate the predictive value of intratumoral and peritumoral radiomics and radiomics nomogram for preoperative lymphovascular invasion (LVI) status and overall survival (OS) in patients with non-small cell lung cancer (NSCLC).
Methods
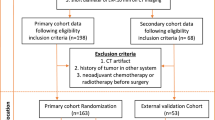
In total, 240 NSCLC patients from our institution were randomly divided into the training cohort (n = 145) and internal validation cohort (n = 95) with a ratio of 6:4, and 65 patients from the Cancer Imaging Archive were enrolled as the external validation cohort. We extracted 1217 CT-based radiomics features from the gross tumor volume (GTV) and gross tumor volume incorporating peritumoral 3, 6, and 9 mm regions (GPTV3, GPTV6, GPTV9). A radiomics nomogram based on clinical independent predictors and radiomics score (Radscore) of the best radiomics model was constructed. The correlation between factors and OS was evaluated with the Kaplan-Meier survival analysis and Cox proportional hazards regression analysis.
Results
Compared with GTV, GPTV3, and GPTV6 radiomics models, GPTV9 radiomics model exhibited better prediction performance with the AUCs of 0.82, 0.75, and 0.67 in the training, internal validation, and external validation cohorts, respectively. In the clinical model, smoking and clinical stage were independent predictors. The nomogram incorporating independent predictors and GPTV9-Radscore was clinically useful, with the AUCs of 0.89, 0.83, and 0.66 in three cohorts. Pathological LVI, GPTV9-Radscore-predicted, and Nomoscore-predicted LVI were associated with poor OS (p < 0.05).
Conclusions
CT-based radiomics nomogram can predict LVI and OS in patients with NSCLC and may help in making personalized treatment strategies before surgery.
Key Points
• Compared with GTV, GPTV 3 , and GPTV 6 radiomics models, GPTV 9 radiomics model showed better prediction performance for LVI status in NSCLC.
• The radiomics nomogram based on GPTV 9 radiomics features and clinical independent predictors could effectively predict LVI status and OS in NSCLC and outperformed the clinical model.
• The radiomics nomogram had a wider scope of clinical application.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under curve
- CT:
-
Computed tomography
- GPTV:
-
Gross peritumoral tumor volume
- GTV:
-
Gross tumor volume
- ICC:
-
Intraclass correlation coefficient
- LASSO:
-
Least absolute shrinkage and selection operator
- LVI:
-
Lymphovascular invasion
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- TCIA:
-
The Cancer Imaging Archive
References
Clark ME, Bedford LE, Young B et al (2018) Lung cancer CT screening: psychological responses in the presence and absence of pulmonary nodules. Lung Cancer 124:160–167
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2012) Global cancer statistics. CA Cancer J Clin 65(2):87–108
Ettinger DS, Wood DE, Aisner DL et al (2021) NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw 19(3):254–266
Sung SY, Kwak YK, Lee SW et al (2018) Lymphovascular invasion increases the risk of nodal and distant recurrence in node-negative stage I-iia non-small-cell lung cancer. Oncology 95(3):156–162
Okada S, Mizuguchi S, Izumi N et al (2017) Prognostic value of the frequency of vascular invasion in stage I non-small cell lung cancer. Gen Thorac Cardiovasc Surg 65:32–39
Shimada Y, Saji H, Kato Y et al (2016) The frequency and prognostic impact of pathological microscopic vascular invasion according to tumor size in non-small cell lung cancer. Chest 149:775–785
Ramnefjell M, Aamelfot C, Helgeland L, Akslen LA (2017) Vascular invasion is an adverse prognostic factor in resected non-small-cell lung cancer. APMIS 125(3):197–206
Shimada Y, Saji H, Yoshida K et al (2012) Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage IA non-small-cell lung cancer after complete surgical resection. J Thorac Oncol 7(8):1263–1270
Wang S, Xu J, Wang R et al (2018) Adjuvant chemotherapy may improve prognosis after resection of stage I lung cancer with lymphovascular invasion. J Thorac Cardiovasc Surg 156(5):2006–2015.e2
Tsutani Y, Miyata Y, Kushitani K, Takeshima Y, Yoshimura M, Okada M (2014) Propensity score-matched analysis of adjuvant chemotherapy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 148:1179–1185
Yun JK, Lee GD, Choi S et al (2020) Comparison of prognostic impact of lymphovascular invasion in stage IA non-small cell lung cancer after lobectomy versus sublobar resection: a propensity score-matched analysis. Lung Cancer 146:105–111
Zhao S, Li F, Guo X et al (2020) New additional scoring formula on the pathological features in stage I lung adenocarcinoma patients: impact on survival. Int J Med Sci 17(13):1871–1878
Ito R, Iwano S, Shimamoto H et al (2017) A comparative analysis of dual-phase dual-energy CT and FDG-PET/CT for the prediction of histopathological invasiveness of non-small cell lung cancer. Eur J Radiol 95:186–191
Gu Q, Feng Z, Liang Q et al (2019) Machine learning-based radiomics strategy for prediction of cell proliferation in non-small cell lung cancer. Eur J Radiol 118:32–37
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ (2017) CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 37(5):1483–1503
Yang G, Nie P, Zhao L et al (2020) 2D and 3D texture analysis to predict lymphovascular invasion in lung adenocarcinoma. Eur J Radiol 129:109–111
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316(8):1324–1331
Wang X, Wan Q, Chen H, Li Y, Li X (2020) Classification of pulmonary lesion based on multiparametric MRI: utility of radiomics and comparison of machine learning methods. Eur Radiol 30(8):4595–4605
Dercle L, Fronheiser M, Lu L et al (2020) Identification of non-small cell lung cancer sensitive to systemic cancer therapies using radiomics. Clin Cancer Res 26(9):2151–2162
Giraud P, Antoine M, Larrouy A et al (2000) Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys 48(4):1015–1024
Liu K, Li K, Wu T et al (2022) Improving the accuracy of prognosis for clinical stage I solid lung adenocarcinoma by radiomics models covering tumor per se and peritumoral changes on CT. Eur Radiol 32(2):1065–1077
Zhang R, Cai Z, Luo Y, Wang Z, Wang W (2021) Preliminary exploration of response the course of radiotherapy for stage III non-small cell lung cancer based on longitudinal CT radiomics features. Eur J Radiol Open 9:100391
Feng Z, Rong P, Cao P et al (2018) Machine learning-based quantitative texture analysis of CT images of small renal masses: differentiation of angiomyolipoma without visible fat from renal cell carcinoma. Eur Radiol 28(4):1625–1633
Ren J, Yuan Y, Qi M, Tao X (2020) Machine learning-based CT texture analysis to predict HPV status in oropharyngeal squamous cell carcinoma: comparison of 2D and 3D segmentation. Eur Radiol 30(12):6858–6866
Chen Y, Xia Y, Tolat PP et al (2021) Comparison of conventional gadoxetate disodium-enhanced MRI features and radiomics signatures with machine learning for diagnosing microvascular invasion. AJR Am J Roentgenol 216(6):1510–1520
Saijo T, Ishii G, Ochiai A et al (2007) Evaluation of extratumoral lymphatic permeation in non-small cell lung cancer as a means of predicting outcome. Lung Cancer 55(1):61–66
Pérez-Morales J, Tunali I, Stringfield O et al (2020) Peritumoral and intratumoral radiomic features predict survival outcomes among patients diagnosed in lung cancer screening. Sci Rep 10(1):10528
Tunali I, Hall LO, Napel S et al (2019) Stability and reproducibility of computed tomography radiomic features extracted from peritumoral regions of lung cancer lesions. Med Phys 46(11):5075–5085
Joyce JA, Pollard JW et al (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252
Jiang T, Song J, Wang X et al (2021) Intratumoral and peritumoral analysis of mammography, tomosynthesis, and multiparametric MRI for predicting Ki-67 level in breast cancer: a radiomics-based study. Mol Imaging Biol. https://doi.org/10.1007/s11307-021-01695-w
Dou TH, Coroller TP, van Griethuysen JJM, Mak RH, Aerts HJWL (2018) Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS One 13(11):e0206108
Wu DM, Deng SH, Zhou J et al (2020) PLEK2 mediates metastasis and vascular invasion via the ubiquitin-dependent degradation of SHIP2 in non-small cell lung cancer. Int J Cancer 146(9):2563–2575
Alwithenani A, Bethune D, Castonguay M et al (2021) Profiling non-small cell lung cancer reveals that PD-L1 is associated with wild type EGFR and vascular invasion, and immunohistochemistry quantification of PD-L1 correlates weakly with RT-qPCR. PLoS One 16(5):e0251080
Li C, Tian Y, Shen Y, Wen B, He Y (2021) Utility of volumetric metabolic parameters on preoperative FDG PET/CT for predicting tumor lymphovascular invasion in non-small cell lung cancer. AJR Am J Roentgenol 217(6):1433–1443
Shimada Y, Ishii G, Hishida T, Yoshida J, Nishimura M, Nagai K (2010) Extratumoral vascular invasion is a significant prognostic indicator and a predicting factor of distant metastasis in non-small cell lung cancer. J Thorac Oncol 5(7):970–975
Sun W, Jiang M, Dang J, Chang P, Yin FF (2018) Effect of machine learning methods on predicting NSCLC overall survival time based on radiomics analysis. Radiat Oncol 13(1):197
Botta F, Raimondi S, Rinaldi L et al (2020) Association of a CT-based clinical and radiomics score of non-small cell lung cancer (NSCLC) with lymph node status and overall survival. Cancers (Basel) 12(6):1432
Funding
This study has received funding from Key Project of Nantong Science and Technology Bureau of China (grant number MS22021047) and Clinical Research Plan of SHDC (grant number SHDC2020CR3080B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Feng Feng.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Shaofeng Duan) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiaoling Chen and JingJing Shao are the first authors.
Supplementary information
ESM 1
(PDF 597 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Q., Shao, J., Xue, T. et al. Intratumoral and peritumoral radiomics nomograms for the preoperative prediction of lymphovascular invasion and overall survival in non-small cell lung cancer. Eur Radiol 33, 947–958 (2023). https://doi.org/10.1007/s00330-022-09109-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09109-3