Abstract
Objectives
Diffuse midline gliomas, H3K27-altered (DMG-A), are malignant gliomas with an unfavorable prognosis. Knowledge of dynamic susceptibility contrast (DSC) MRI findings and imaging differences with high-grade midline glioma without H3K27 alteration (DMG-W) has been limited. We compared the DSC, ADC, and conventional MRI findings between DMG-A and DMG-W.
Methods
In this single institutional retrospective study, the electronic database of our hospital between June 2015 and May 2021 was searched. Twenty and 17 patients with DMG-A (median, 13 years; range, 3–52 years; 11 females) and DMG-W (median, 40 years; 7–73 years; 9 females), respectively, were found. Normalized relative cerebral blood flow (nrCBF) and normalized corrected relative cerebral blood volume (ncrCBV); normalized maximum, mean, and minimum ADC values; and the prevalence of T2-FLAIR mismatch sign were compared between the two groups using Mann–Whitney U tests and Fisher’s exact test.
Results
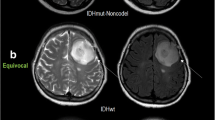
The nrCBF and ncrCBV were significantly lower in DMG-A compared with DMG-W (nrCBF: median 0.88 [range, 0.19–2.67] vs. 1.47 [range, 0.57–4.90] (p < 0.001); ncrCBV: 1.17 [0.20–2.67] vs. 1.56 [0.60–4.03] (p = 0.008)). Normalized maximum ADC (nADCmax) was significantly higher in DMG-A (median 2.37 [1.25–3.98] vs. 1.95 [1.23–2.77], p = 0.02). T2-FLAIR mismatch sign was significantly more common in DMG-A (11/20 (55.0%) vs. 1/17 (5.9%), p = 0.0017). When at least two of nrCBF < 1.11, nADCmax ≥ 2.48, and T2-FLAIR mismatch sign were positive, the diagnostic performance was the highest with accuracy of 0.81.
Conclusion
DSC-MRI parameters, ADC values, and the T2-FLAIR mismatch sign are useful to differentiate between DMG-A and DMG-W.
Key Points
• Diffuse midline glioma, H3K27-altered (DMG-A), showed a significantly lower normalized relative cerebral blood flow and volume compared with H3K27-wild-type counterparts (DMG-W).
• T2-fluid-attenuated inversion recovery (FLAIR) mismatch sign was significantly more frequent in DMG-A compared to DMG-W.
• Indicators that combined DSC parameters, ADC values, and T2-FLAIR mismatch sign, with or without age, are useful to distinguish the two tumors.




Similar content being viewed by others
Abbreviations
- AIF:
-
Arterial input function
- DMG-A:
-
Diffuse midline glioma, H3K27-altered
- DMG-W:
-
Midline high-grade glioma without H3K27 alteration
- DSC:
-
Dynamic susceptibility contrast
- nADCmax /mean/min :
-
Normalized maximum/mean/minimum ADC
- ncrCBV:
-
Normalized corrected relative cerebral blood volume
- nrCBF:
-
Normalized relative cerebral blood flow
- ROI:
-
Region-of-interest
- WHO:
-
World Health Organization
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. https://doi.org/10.1093/neuonc/noab106
Sievers P, Sill M, Schrimpf D et al (2021) A subset of pediatric-type thalamic gliomas share a distinct DNA methylation profile, H3K27me3 loss and frequent alteration of EGFR. Neuro Oncol 23:34–43
Cooney TM, Lubanszky E, Prasad R, Hawkins C, Mueller S (2020) Diffuse midline glioma: review of epigenetics. J Neurooncol 150:27–34
Aboian MS, Solomon DA, Felton E et al (2017) Imaging characteristics of pediatric diffuse midline gliomas with histone H3 K27M mutation. AJNR Am J Neuroradiol 38:795–800
Mackay A, Burford A, Carvalho D et al (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32:520-537.e5
Aboian MS, Tong E, Solomon DA et al (2019) Diffusion characteristics of pediatric diffuse midline gliomas with histone H3–K27M mutation using apparent diffusion coefficient histogram analysis. AJNR Am J Neuroradiol 40:1804–1810
Schulte JD, Buerki RA, Lapointe S et al (2020) Clinical, radiologic, and genetic characteristics of histone H3 K27M-mutant diffuse midline gliomas in adults. Neurooncol Adv 2:vdaa142
Leach JL, Roebker J, Schafer A et al (2020) MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the International DIPG Registry. Neuro Oncol 22:1647–1657
Thust S, Micallef C, Okuchi S et al (2021) Imaging characteristics of H3 K27M histone-mutant diffuse midline glioma in teenagers and adults. Quant Imaging Med Surg 11:43–56
Morana G, Tortora D, Staglianò S et al (2018) Pediatric astrocytic tumor grading: comparison between arterial spin labeling and dynamic susceptibility contrast MRI perfusion. Neuroradiology 60:437–446
Nguyen TB, Cron GO, Perdrizet K et al (2015) Comparison of the diagnostic accuracy of DSC- and dynamic contrast-enhanced MRI in the preoperative grading of astrocytomas. AJNR Am J Neuroradiol 36:2017–2022
Löbel U, Sedlacik J, Reddick WE et al (2011) Quantitative diffusion-weighted and dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging analysis of T2 hypointense lesion components in pediatric diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol 32:315–322
Sedlacik J, Winchell A, Kocak M, Loeffler RB, Broniscer A, Hillenbrand CM (2013) MR imaging assessment of tumor perfusion and 3D segmented volume at baseline, during treatment, and at tumor progression in children with newly diagnosed diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol 34:1450–1455
Patel SH, Poisson LM, Brat DJ et al (2017) T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res 23:6078–6085
Juratli TA, Tummala SS, Riedl A et al (2019) Radiographic assessment of contrast enhancement and T2/FLAIR mismatch sign in lower grade gliomas: correlation with molecular groups. J Neurooncol 141:327–335
Chen H, Hu W, He H, Yang Y, Wen G, Lv X (2019) Noninvasive assessment of H3 K27M mutational status in diffuse midline gliomas by using apparent diffusion coefficient measurements. Eur J Radiol 114:152–159
Mouridsen K, Christensen S, Gyldensted L, Ostergaard L (2006) Automatic selection of arterial input function using cluster analysis. Magn Reson Med 55:524–531
Meyronet D, Esteban-Mader M, Bonnet C et al (2017) Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol 19:1127–1134
Ebrahimi A, Skardelly M, Schuhmann MU et al (2019) High frequency of H3 K27M mutations in adult midline gliomas. J Cancer Res Clin Oncol 145:839–850
Schreck KC, Ranjan S, Skorupan N et al (2019) Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol 143:87–93
Chi AS, Tarapore RS, Hall MD et al (2019) Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol 145:97–105
Broen MPG, Smits M, Wijnenga MMJ et al (2018) The T2-FLAIR mismatch sign as an imaging marker for non-enhancing IDH-mutant, 1p/19q-intact lower-grade glioma: a validation study. Neuro Oncol 20:1393–1399
Fujita Y, Nagashima H, Tanaka K et al (2021) The histopathologic and radiologic features of T2-FLAIR mismatch sign in IDH-Mutant 1p/19q non-codeleted astrocytomas. World Neurosurg 149:e253–e260
Deguchi S, Oishi T, Mitsuya K et al (2020) Clinicopathological analysis of T2-FLAIR mismatch sign in lower-grade gliomas. Sci Rep 10:10113
Aliotta E, Dutta SW, Feng X et al (2020) Automated apparent diffusion coefficient analysis for genotype prediction in lower grade glioma: association with the T2-FLAIR mismatch sign. J Neurooncol 149:325–335
Onishi S, Amatya VJ, Kolakshyapati M et al (2020) T2-FLAIR mismatch sign in dysembryoplasticneuroepithelial tumor. Eur J Radiol 126:108924
Johnson DR, Kaufmann TJ, Patel SH, Chi AS, Snuderl M, Jain R (2019) There is an exception to every rule-T2-FLAIR mismatch sign in gliomas. Neuroradiology 61:225–227
Draaisma K, Wijnenga MM, Weenink B et al (2015) PI3 kinase mutations and mutational load as poor prognostic markers in diffuse glioma patients. Acta Neuropathol Commun 3:88
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Harshbarger TB, Song AW (2006) Endogenous functional CBV contrast revealed by diffusion weighting. NMR Biomed 19:1020–1027
Acknowledgements
The authors thank Ms. Nancy Dudek, an MRI Technologist at University of Michigan Health System, for her technical support. The authors thank Dr. Takeru Q Suyama at National Institute of Informatics, for his advice on statistics.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Toshio Moritani, Division of Neuroradiology, Department of Radiology, Michigan Medicine, 1500E Medical Center Drive Ann Arbor, MI 48109, United States.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurokawa, R., Kurokawa, M., Baba, A. et al. Dynamic susceptibility contrast-MRI parameters, ADC values, and the T2-FLAIR mismatch sign are useful to differentiate between H3-mutant and H3-wild-type high-grade midline glioma. Eur Radiol 32, 3672–3682 (2022). https://doi.org/10.1007/s00330-021-08476-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08476-7