Abstract
Objectives
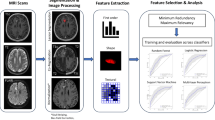
The identification of viable tumor after stereotactic radiosurgery (SRS) is important for future targeted therapy. This study aimed to determine whether tumor habitat on structural and physiologic MRI can distinguish viable tumor from radiation necrosis of brain metastases after SRS.
Method
Multiparametric contrast-enhanced T1- and T2-weighted imaging, apparent diffusion coefficient (ADC), and cerebral blood volume (CBV) were obtained from 52 patients with 69 metastases, showing enlarging enhancing masses after SRS. Voxel-wise clustering identified three structural MRI habitats (enhancing, solid low-enhancing, and nonviable) and three physiologic MRI habitats (hypervascular cellular, hypovascular cellular, and nonviable). Habitat-based predictors for viable tumor or radiation necrosis were identified by logistic regression. Performance was validated using the area under the curve (AUC) of the receiver operating characteristics curve in an independent dataset with 24 patients.
Results
None of the physiologic MRI habitats was indicative of viable tumor. Viable tumor was predicted by a high-volume fraction of solid low-enhancing habitat (low T2-weighted and low CE-T1-weighted values; odds ratio [OR] 1.74, p <.001) and a low-volume fraction of nonviable tissue habitat (high T2-weighted and low CE-T1-weighted values; OR 0.55, p <.001). Combined structural MRI habitats yielded good discriminatory ability in both development (AUC 0.85, 95% confidence interval [CI]: 0.77–0.94) and validation sets (AUC 0.86, 95% CI:0.70–0.99), outperforming single ADC (AUC 0.64) and CBV (AUC 0.58) values. The site of progression matched with the solid low-enhancing habitat (72%, 8/11).
Conclusion
Solid low-enhancing and nonviable tissue habitats on structural MRI can help to localize viable tumor in patients with brain metastases after SRS.
Key Points
• Structural MRI habitats helped to differentiate viable tumor from radiation necrosis.
• Solid low-enhancing habitat was most helpful to find viable tumor.
• Providing spatial information, the site of progression matched with solid low-enhancing habitat.




Similar content being viewed by others
Abbreviations
- CEL:
-
Contrast-enhancing lesion
- SRS:
-
Stereotactic radiosurgery
References
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL (2011) A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol 32:1885–1892
Di Chiro G, Oldfield E, Wright DC et al (1988) Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Roentgenol 150:189–197
Belka C, Budach W, Kortmann RD, Bamberg M (2001) Radiation induced CNS toxicity--molecular and cellular mechanisms. Br J Cancer 85:1233–1239
Yoshii Y (2008) Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 25:51–58
Rahmathulla G, Marko NF, Weil RJ (2013) Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci 20:485–502
Zeng YD, Liao H, Qin T et al (2015) Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget 6:8366–8376
Alic L, Niessen WJ, Veenland JF (2014) Quantification of heterogeneity as a biomarker in tumor imaging: a systematic review. PLoS One 9:e110300
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213
O'Connor JPB, Rose CJ, Waterton JC, Carano RAD, Parker GJM, Jackson A (2015) Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res 21:249–257
Lee J, Narang S, Martinez J, Rao G, Rao A (2015) Spatial habitat features derived from multiparametric magnetic resonance imaging data are associated with molecular subtype and 12-month survival status in glioblastoma multiforme. PLoS One 10:e0136557
Kanungo T, Mount DM, Netanyahu NS, Piatko CD, Silverman R, Wu AY (2002) An efficient k-means clustering algorithm: analysis and implementation. IEEE Trans Pattern Anal Mach Intell 24:881–892
Vannier MW, Butterfield RL, Jordan D, Murphy WA, Levitt RG, Gado M (1985) Multispectral analysis of magnetic-resonance images. Radiology 154:221–224
Gaustad JV, Benjaminsen IC, Graff BA, Brurberg KG, Ruud EBM, Rofstad EK (2005) Intratumor heterogeneity in blood perfusion in orthotopic human melanoma xenografts assessed by dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 21:792–800
Checkley D, Tessier JJ, Kendrew J, Waterton JC, Wedge SR (2003) Use of dynamic contrast-enhanced MRI to evaluate acute treatment with ZD6474, a VEGF signalling inhibitor, in PC-3 prostate tumours. Br J Cancer 89:1889–1895
Miller JA, Bennett EE, Xiao R et al (2016) Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys 96:1060–1069
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278
Shah R, Vattoth S, Jacob R et al (2012) Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics 32:1343–1359
Levin VA, Bidaut L, Hou P et al (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487–1495
Reinhold JC, Dewey BE, Carass A, Prince JL (2019) Evaluating the impact of intensity normalization on MR image synthesis. Proc SPIE Int Soc Opt Eng 10949:109493H
Jain R (2011) Perfusion CT imaging of brain tumors: an overview. AJNR Am J Neuroradiol 32:1570–1577
Gull SF (1988) Bayesian inductive inference and maximum entropy. In: Erickson GJ, Smith CR (eds) Maximum-entropy and Bayesian methods in science and engineering: foundations. Springer Netherlands, Dordrecht, pp 53–74
Genders TS, Spronk S, Stijnen T, Steyerberg EW, Lesaffre E, Hunink MG (2012) Methods for calculating sensitivity and specificity of clustered data: a tutorial. Radiology 265:910–916
McCulloch CE, Neuhaus JM (2014) Generalized linear mixed models. Statistics Reference Online, Wiley StatsRef
Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med 13:1
Lohmann P, Kocher M, Ceccon G et al (2018) Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin 20:537–542
Karami E, Soliman H, Ruschin M et al (2019) Quantitative MRI biomarkers of stereotactic radiotherapy outcome in brain metastasis. Sci Rep 9:19830
Huang CY, Lee CC, Yang HC et al (2020) Radiomics as prognostic factor in brain metastases treated with Gamma Knife radiosurgery. J Neurooncol 146:439–449
Dequesada IM, Quisling RG, Yachnis A, Friedman WA (2008) Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 63:898–903 discussion 904
Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD (2010) T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 66:486–491 discussion 491-482
Stockham AL, Tievsky AL, Koyfman SA et al (2012) Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol 109:149–158
Ahn SJ, Park M, Bang S et al (2018) Apparent diffusion coefficient histogram in breast cancer brain metastases may predict their biological subtype and progression. Sci Rep 8:9947
Jung WS, Park CH, Hong CK, Suh SH, Ahn SJ (2018) Diffusion-weighted imaging of brain metastasis from lung cancer: correlation of MRI parameters with the histologic type and gene mutation status. AJNR Am J Neuroradiol 39:273–279
Knitter JR, Erly WK, Stea BD et al (2018) Interval change in diffusion and perfusion MRI parameters for the assessment of pseudoprogression in cerebral metastases treated with stereotactic radiation. AJR Am J Roentgenol 211:168–175
Huang C-F, Chou H-H, Tu H-T, Yang M-S, Lee J-K, Lin L-Y (2008) Diffusion magnetic resonance imaging as an evaluation of the response of brain metastases treated by stereotactic radiosurgery. Surg Neurol 69:62–68
Lee CC, Wintermark M, Xu Z, Yen CP, Schlesinger D, Sheehan JP (2014) Application of diffusion-weighted magnetic resonance imaging to predict the intracranial metastatic tumor response to gamma knife radiosurgery. J Neurooncol 118:351–361
Hoefnagels FW, Lagerwaard FJ, Sanchez E et al (2009) Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 256:878
Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S (2009) Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 30:367–372
Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH (2015) Review of cranial radiotherapy-induced vasculopathy. J Neurooncol 122:421–429
Cristante E, McArthur S, Mauro C et al (2013) Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci U S A 110:832–841
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (grant numbers NRF-2020R1A2B5B01001707 and NRF-2020R1A2C4001748).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ho Sung Kim.
Conflict of interest
One of the authors of this manuscript (NakYoung Kim) is an employee of DYNAPEX LLC. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise (Seo Young Park, 8 years of experience as a statistician).
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1039 kb)
Rights and permissions
About this article
Cite this article
Lee, D.H., Park, J.E., Kim, N. et al. Tumor habitat analysis by magnetic resonance imaging distinguishes tumor progression from radiation necrosis in brain metastases after stereotactic radiosurgery. Eur Radiol 32, 497–507 (2022). https://doi.org/10.1007/s00330-021-08204-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08204-1