Abstract
Objective
To investigate the correlation of intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) parameters with the expression of HIF-1α in soft tissue sarcoma (STS).
Methods
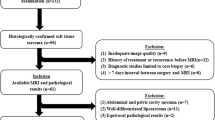
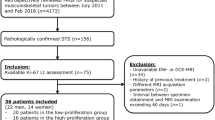
This prospective study was approved by the institutional ethics committee. Written informed consent was obtained from all patients. Forty patients with STS who underwent 3.0 T MRI, including IVIM and DKI, were included in the study. Standard apparent diffusion coefficient (ADC), true ADC (Dslow), pseudo ADC (Dfast), perfusion fraction (f), mean kurtosis (MK), and mean diffusivity (MD) of each lesion were independently analyzed by two observers. An MRI-pathology control method was used to ensure correspondence between the MRI slices and the pathological sections. Spearman analysis, independent sample t test, Mann-Whitney U test, chi-squared test, and receiver operating characteristic (ROC) curve analysis were performed.
Results
Dslow and MD values showed a negative correlation with HIF-1α expression (r = − 0.469, − 0.588). MK and f values showed a positive correlation with HIF-1α expression (r = 0.779, 0.572). Dslow, MD, MK, and f values showed significant differences between the high- and low-expression groups. The MK value showed the best diagnostic ability. The optimal cut-off MK value of 0.604 was associated with 78.3% sensitivity and 88.2% specificity (area under the curve, 0.867).
Conclusions
This preliminary study demonstrated the association of IVIM and DKI parameters with the expression of HIF-1α in STS.
Key Points
• IVIM and DKI parameters are correlated with the expression of HIF-1α in STS.
• The MRI-pathology control method can be used in clinical studies to ensure correspondence between MRI slices and pathology sections.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DKI:
-
Diffusion kurtosis imaging
- DWI:
-
Diffusion-weighted imaging
- EPI:
-
Echo-planar imaging
- FNCLCC:
-
Fédération Nationale des Centres de Lutte Contre le Cancer
- HIF:
-
Hypoxia-inducible factors
- IVIM:
-
Intravoxel incoherent motion
- ROI:
-
Region of interest
- STS:
-
Soft tissue sarcoma
References
von Mehren M, Randall RL, Benjamin RS et al (2018) Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16:536–563
Bosco MC, D'Orazi G, Del Bufalo D (2020) Targeting hypoxia in tumor: a new promising therapeutic strategy. J Exp Clin Cancer Res 39:8
Young RM, Simon MC (2012) Untuning the tumor metabolic machine: HIF-alpha: pro- and antitumorigenic? Nat Med 18:1024–1025
Cheng W, Cheng Z, Yang Z, Xing D, Zhang M (2019) Upregulation of hypoxia-inducible factor 1alpha mRNA expression was associated with poor prognosis in patients with hepatocellular carcinoma. Onco Targets Ther 12:6285–6296
Guo Y, Xiao Z, Yang L et al (2020) Hypoxiainducible factors in hepatocellular carcinoma (Review). Oncol Rep 43:3–15
Ranasinghe WK, Baldwin GS, Shulkes A, Bolton D, Patel O (2014) Normoxic regulation of HIF-1alpha in prostate cancer. Nat Rev Urol 11:419
Potharaju M, Mathavan A, Mangaleswaran B et al (2019) Clinicopathological analysis of HIF-1alpha and TERT on survival outcome in glioblastoma patients: a prospective, single institution study. J Cancer 10:2397–2406
Gruber G, Greiner RH, Hlushchuk R et al (2004) Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res 6:R191–R198
Gong L, Zhang W, Zhou J et al (2013) Prognostic value of HIFs expression in head and neck cancer: a systematic review. PLoS One 8:e75094
Shintani K, Matsumine A, Kusuzaki K et al (2006) Expression of hypoxia-inducible factor (HIF)-1alpha as a biomarker of outcome in soft-tissue sarcomas. Virchows Arch 449:673–681
Kim JI, Choi KU, Lee IS et al (2015) Expression of hypoxic markers and their prognostic significance in soft tissue sarcoma. Oncol Lett 9:1699–1706
Forker L, Gaunt P, Sioletic S et al (2018) The hypoxia marker CAIX is prognostic in the UK phase III VorteX-Biobank cohort: an important resource for translational research in soft tissue sarcoma. Br J Cancer 118:698–704
Rot S, Taubert H, Bache M et al (2018) Low HIF-1alpha and low EGFR mRNA expression significantly associate with poor survival in soft tissue sarcoma patients; the proteins react differently. Int J Mol Sci 19:3842. https://doi.org/10.3390/ijms19123842
Li Y, Zhang W, Li S, Tu C (2016) Prognosis value of hypoxia-inducible factor-1alpha expression in patients with bone and soft tissue sarcoma: a meta-analysis. Springerplus 5:1370
Eisinger-Mathason TS, Zhang M, Qiu Q et al (2013) Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov 3:1190–1205
Misra RM, Bajaj MS, Kale VP (2012) Vasculogenic mimicry of HT1080 tumour cells in vivo: critical role of HIF-1alpha-neuropilin-1 axis. PLoS One 7:e50153
Birgin E, Yang C, Hetjens S, Reissfelder C, Hohenberger P, Rahbari NN (2020) Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: a systematic review and meta-analysis. Cancer. https://doi.org/10.1002/cncr.32735
Liang J, Cheng Q, Huang J et al (2019) Monitoring tumour microenvironment changes during anti-angiogenesis therapy using functional MRI. Angiogenesis 22:457–470
Liu M, Guo X, Wang S et al (2013) BOLD-MRI of breast invasive ductal carcinoma: correlation of R2* value and the expression of HIF-1alpha. Eur Radiol 23:3221–3227
Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL (2014) Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol 16:280–291
Lindgren A, Anttila M, Rautiainen S et al (2019) Dynamic contrast-enhanced perfusion parameters in ovarian cancer: good accuracy in identifying high HIF-1alpha expression. PLoS One 14:e0221340
Ma T, Yang S, Jing H et al (2018) Apparent diffusion coefficients in prostate cancer: correlation with molecular markers Ki-67, HIF-1alpha and VEGF. NMR Biomed 31
Hu Y, E H, Yu X et al (2018) Correlation of quantitative parameters of magnetic resonance perfusion-weighted imaging with vascular endothelial growth factor, microvessel density and hypoxia-inducible factor-1alpha in nasopharyngeal carcinoma: evaluation on radiosensitivity study. Clin Otolaryngol 43:425–433
Kakkad S, Krishnamachary B, Jacob D et al (2019) Molecular and functional imaging insights into the role of hypoxia in cancer aggression. Cancer Metastasis Rev 38:51–64
Yuan Y, Zeng D, Liu Y et al (2020) DWI and IVIM are predictors of Ki67 proliferation index: direct comparison of MRI images and pathological slices in a murine model of rhabdomyosarcoma. Eur Radiol 30:1334–1341
Li X, Wu S, Li D et al (2019) Intravoxel incoherent motion combined with dynamic contrast-enhanced perfusion MRI of early cervical carcinoma: correlations between multimodal parameters and HIF-1alpha expression. J Magn Reson Imaging 50:918–929
Dhakal HP, Nesland JM, Forsund M, Trope CG, Holm R (2013) Primary tumor vascularity, HIF-1alpha and VEGF expression in vulvar squamous cell carcinomas: their relationships with clinicopathological characteristics and prognostic impact. BMC Cancer 13:506
van Nimwegen LWE, Mavinkurve-Groothuis AMC, de Krijger RR et al (2020) MR imaging in discriminating between benign and malignant paediatric ovarian masses: a systematic review. Eur Radiol 30:1166–1181
Meng X, Li H, Kong L et al (2016) MRI in rectal cancer: correlations between MRI features and molecular markers Ki-67, HIF-1alpha, and VEGF. J Magn Reson Imaging 44:594–600
Huang Z, Xu X, Meng X et al (2015) Correlations between ADC values and molecular markers of Ki-67 and HIF-1alpha in hepatocellular carcinoma. Eur J Radiol 84:2464–2469
Swartz JE, Driessen JP, van Kempen PMW et al (2018) Influence of tumor and microenvironment characteristics on diffusion-weighted imaging in oropharyngeal carcinoma: a pilot study. Oral Oncol 77:9–15
Liang J, Ma R, Chen H et al (2019) Detection of hyperacute reactions of desacetylvinblastine monohydrazide in a xenograft model using intravoxel incoherent motion DWI and R2* mapping. AJR Am J Roentgenol 212:717–726. https://doi.org/10.2214/AJR.18.20517
Lee JH, Yoon YC, Seo SW, Choi YL, Kim HS (2020) Soft tissue sarcoma: DWI and DCE-MRI parameters correlate with Ki-67 labeling index. Eur Radiol 30:914–924
Patra K, Jana S, Sarkar A, Mandal DP, Bhattacharjee S (2019) The inhibition of hypoxia-induced angiogenesis and metastasis by cinnamaldehyde is mediated by decreasing HIF-1alpha protein synthesis via PI3K/Akt pathway. Biofactors 45:401–415
Dyvorne HA, Galea N, Nevers T et al (2013) Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters--a pilot study. Radiology 266:920–929
Liu C, Wang K, Chan Q et al (2016) Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol 26:3888–3898
Lee EY, Yu X, Chu MM et al (2014) Perfusion and diffusion characteristics of cervical cancer based on intraxovel incoherent motion MR imaging-a pilot study. Eur Radiol 24:1506–1513
Winfield JM, Orton MR, Collins DJ et al (2017) Separation of type and grade in cervical tumours using non-mono-exponential models of diffusion-weighted MRI. Eur Radiol 27:627–636
Nogueira L, Brandao S, Matos E et al (2014) Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol 24:1197–1203
Wu G, Liu X, Xiong Y, Ran J, Li X (2018) Intravoxel incoherent motion and diffusion kurtosis imaging for discriminating soft tissue sarcoma from vascular anomalies. Medicine (Baltimore) 97:e13641
Zhao F, Ahlawat S, Farahani SJ et al (2014) Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology 272:192–201
Chhabra A, Ashikyan O, Slepicka C et al (2019) Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur Radiol 29:4485–4494
Crombe A, Marcellin PJ, Buy X et al (2019) Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology 291:710–721
Funding
This study has received funding from the National Natural Science Foundation of China (81771804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shaowu Wang, MD, PHD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• case-control study/diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 4961 kb)
Rights and permissions
About this article
Cite this article
Li, X., Yang, L., Wang, Q. et al. Soft tissue sarcomas: IVIM and DKI correlate with the expression of HIF-1α on direct comparison of MRI and pathological slices. Eur Radiol 31, 4669–4679 (2021). https://doi.org/10.1007/s00330-020-07526-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07526-w