Abstract
Objectives
To analyze the effect of preoperative body composition on survival in patients with pancreatic cancer following pancreaticoduodenectomy (PD).
Methods
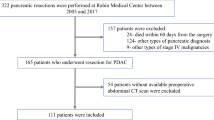
Between October 2005 and August 2018, 116 patients (68 men, 48 women, mean age 66.2 ± 11.9 years) diagnosed with pancreatic adenocarcinoma following PD were retrospectively enrolled. The preoperative CT on vertebral level L3 was assessed for total abdominal muscle area (TAMA), visceral adipose tissue area (VAT), subcutaneous adipose tissue area (SAT), and mean skeletal muscle attenuation (SMD). The clinical data and pathological findings of tumors were collected. The impact of these factors on disease-free survival (DFS) and overall survival (OS) was evaluated by the Kaplan–Meier method and by univariable and multivariable Cox proportional hazards models.
Results
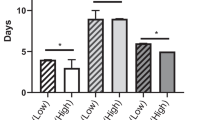
The 3-year DFS and OS rates were 8% and 25%, respectively. Of 116 patients, 20 (17.2%), 3 (2.6%), and 46 (39.7%) patients were classified as having sarcopenia, sarcopenic obesity, and myosteatosis, respectively. The VAT–TAMA ratio (1.2 ± 0.7 vs 0.9 ± 0.5, p = 0.01) and the visceral to subcutaneous adipose tissue area ratio (1.3 ± 0.7 vs 0.9 ± 0.5, p = 0.04) were higher in sarcopenic patients than in the nonsarcopenic group. Preoperative sarcopenia and sarcopenic obesity were associated with shorter OS (p = 0.012 and p = 0.041, respectively), but not shorter DFS. Myosteatosis was neither associated with DFS nor OS. On multivariable analysis, sarcopenia was the only significant prognostic factor for OS (p = 0.039).
Conclusions
Preoperative sarcopenia assessed by CT is a poor prognostic factor for OS in pancreatic cancer patients after PD.
Key Points
• Sarcopenia and sarcopenic obesity can be evaluated by abdominal CT on L3 level.
• Patients with diabetes mellitus (DM) had lower sex-standardized subcutaneous adipose tissue area index and skeletal muscle density and higher visceral to subcutaneous adipose tissue area ratio than did those without DM.
• Preoperative sarcopenia, sarcopenic obesity, and new-onset diabetes mellitus may predict poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy.



Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- CA 19-9:
-
Carbohydrate antigen 19-9
- CI:
-
Confidence interval
- DFS:
-
Disease-free survival
- DM:
-
Diabetes mellitus
- HR:
-
Hazard ratio
- HU:
-
Hounsfield unit
- OS:
-
Overall survival
- PD:
-
Pancreaticoduodenectomy
- SAT:
-
Subcutaneous adipose tissue area
- SATI:
-
Subcutaneous adipose tissue area index
- SMD:
-
Skeletal muscle density
- SMI:
-
Skeletal muscle index
- TAMA:
-
Total abdominal muscle area
- VAT:
-
Visceral adipose tissue area
- VATI:
-
Visceral adipose tissue area index
- VSR:
-
Visceral to subcutaneous adipose tissue area ratio
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Ryan DP, Hong TS, Bardeesy N (2014) Pancreatic adenocarcinoma. N Engl J Med 371:1039–1049
Conroy T, Hammel P, Hebbar M et al (2018) FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395–2406
Strobel O, Neoptolemos J, Jager D, Buchler MW (2019) Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 16:11–26
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Hilmi M, Jouinot A, Burns R et al (2019) Body composition and sarcopenia: the next-generation of personalized oncology and pharmacology? Pharmacol Ther 196:135–159
Chen LK, Woo J, Assantachai P et al (2020) Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21:300–307 e302
Tosato M, Marzetti E, Cesari M et al (2017) Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res 29:19–27
Sanabria SJ, Martini K, Freystatter G et al (2019) Speed of sound ultrasound: a pilot study on a novel technique to identify sarcopenia in seniors. Eur Radiol 29:3–12
Faron A, Pieper CC, Schmeel FC et al (2019) Fat-free muscle area measured by magnetic resonance imaging predicts overall survival of patients undergoing radioembolization of colorectal cancer liver metastases. Eur Radiol 29:4709–4717
Zopfs D, Theurich S, Grosse Hokamp N et al (2020) Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol 30:1701–1708
Lee K, Shin Y, Huh J et al (2019) Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol 20:205–217
Albano D, Messina C, Vitale J, Sconfienza LM (2020) Imaging of sarcopenia: old evidence and new insights. Eur Radiol 30:2199–2208
Prado CM, Birdsell LA, Baracos VE (2009) The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 3:269–275
Chan MY, Chok KSH (2019) Sarcopenia in pancreatic cancer - effects on surgical outcomes and chemotherapy. World J Gastrointest Oncol 11:527–537
Mintziras I, Miligkos M, Wachter S, Manoharan J, Maurer E, Bartsch DK (2018) Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg 59:19–26
Choi MH, Yoon SB, Lee K et al (2018) Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle 9:326–334
Peng P, Hyder O, Firoozmand A et al (2012) Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 16:1478–1486
Amini N, Spolverato G, Gupta R et al (2015) Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 19:1593–1602
Choi Y, Oh DY, Kim TY et al (2015) Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One 10:e0139749
Pecorelli N, Carrara G, De Cobelli F et al (2016) Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg 103:434–442
Ninomiya G, Fujii T, Yamada S et al (2017) Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg 39:45–51
Sugimoto M, Farnell MB, Nagorney DM et al (2018) Decreased skeletal muscle volume is a predictive factor for poorer survival in patients undergoing surgical resection for pancreatic ductal adenocarcinoma. J Gastrointest Surg 22:831–839
van Dijk DP, Bakens MJ, Coolsen MM et al (2017) Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle 8:317–326
Pannala R, Basu A, Petersen GM, Chari ST (2009) New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 10:88–95
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Fedorov A, Beichel R, Kalpathy-Cramer J et al (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323–1341
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 85:115–122
Okumura S, Kaido T, Hamaguchi Y et al (2017) Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann Surg Oncol 24:3732–3740
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Yamane H, Abe T, Amano H et al (2018) Visceral adipose tissue and skeletal muscle index distribution predicts severe pancreatic fistula development after pancreaticoduodenectomy. Anticancer Res 38:1061–1066
Ozturk ZA, Kul S, Turkbeyler IH, Sayiner ZA, Abiyev A (2018) Is increased neutrophil lymphocyte ratio remarking the inflammation in sarcopenia? Exp Gerontol 110:223–229
Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z (2018) Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta 479:181–189
Abe T, Nakata K, Kibe S et al (2018) Prognostic value of preoperative nutritional and immunological factors in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol 25:3996–4003
Gruber ES, Jomrich G, Tamandl D, Gnant M, Schindl M, Sahora K (2019) Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One 14:e0215915
Jang M, Park HW, Huh J et al (2019) Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI. Eur Radiol 29:2417–2425
Prado CMM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Aggarwal G, Kamada P, Chari ST (2013) Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas 42:198–201
Aggarwal G, Rabe KG, Petersen GM, Chari ST (2012) New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology 12:156–161
Lv X, Qiao W, Leng Y, Wu L, Zhou Y (2017) Impact of diabetes mellitus on clinical outcomes of pancreatic cancer after surgical resection: a systematic review and meta-analysis. PLoS One 12:e0171370
Lee S, Hwang HK, Kang CM, Lee WJ (2018) Adverse oncologic impact of new-onset diabetes mellitus on recurrence in resected pancreatic ductal adenocarcinoma: a comparison with long-standing and non–diabetes mellitus patients. Pancreas 47:816–822
Preis SR, Massaro JM, Robins SJ et al (2010) Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity (Silver Spring) 18:2191–2198
Fukuda T, Bouchi R, Takeuchi T et al (2018) Ratio of visceral-to-subcutaneous fat area predicts cardiovascular events in patients with type 2 diabetes. J Diabetes Investig 9:396–402
Gupta P, Lanca C, Gan ATL et al (2019) The association between body composition using dual energy X-ray absorptiometry and type-2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep 9:12634
Abe Y, Urakami T, Hara M et al (2019) The characteristics of abdominal fat distribution in Japanese adolescents with type 2 diabetes mellitus. Diabetes Metab Syndr Obes 12:2281–2288
Funding
This study has received funding from the National Taiwan University Hospital and Industrial Technology Research Institute cooperation research project (PC1233).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Bang-Bin Chen, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Bang-Bin Chen, MD, has significant statistical expertise, and no complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, YC., Wu, CH., Tien, YW. et al. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol 31, 2472–2481 (2021). https://doi.org/10.1007/s00330-020-07294-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07294-7