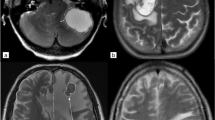
Abstract
Objectives
Malignant tumours consist of biologically heterogeneous components; identifying and stratifying those various subregions is an important research topic. We aimed to show the effectiveness of an intratumour partitioning method using clustering to identify highly aggressive tumour subregions, determining prognosis based on pre-treatment PET and DWI in stage IV lung adenocarcinoma.
Methods
Eighteen patients who underwent both baseline PET and DWI were recruited. Pre-treatment imaging of SUV and ADC values were used to form intensity vectors within manually specified ROIs. We applied k-means clustering to intensity vectors to yield distinct subregions, then chose the subregion that best matched the criteria for high SUV and low ADC to identify tumour subregions with high aggressiveness. We stratified patients into high- and low-risk groups based on subregion volume with high aggressiveness and conducted survival analyses. This approach is referred to as the partitioning approach. For comparison, we computed tumour subregions with high aggressiveness without clustering and repeated the described procedure; this is referred to as the voxel-wise approach.
Results
The partitioning approach led to high-risk (median SUVmax = 14.25 and median ADC = 1.26x10-3 mm2/s) and low-risk (median SUVmax = 14.64 and median ADC = 1.09x10-3 mm2/s) subgroups. Our partitioning approach identified significant differences in survival between high- and low-risk subgroups (hazard ratio, 4.062, 95% confidence interval, 1.21 – 13.58, p-value: 0.035). The voxel-wise approach did not identify significant differences in survival between high- and low-risk subgroups (p-value: 0.325).
Conclusion
Our partitioning approach identified intratumour subregions that were predictors of survival.
Key Points
• Multimodal imaging of PET and DWI is useful for assessing intratumour heterogeneity.
• Data-driven clustering identified subregions which might be highly aggressive for lung adenocarcinoma.
• The data-driven partitioning results might be predictors of survival.




Similar content being viewed by others
Abbreviations
- DCR:
-
Disease control rate
- HR:
-
Hazard ratio
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PFS:
-
Progression free survival
References
O’Connor JPB, Rose CJ, Waterton JC et al (2015) Imaging intratumor heterogeneity: Role in therapy response, resistance, and clinical outcome. Clin Cancer Res 21:249–257. https://doi.org/10.1158/1078-0432.CCR-14-0990
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Shipitsin M, Campbell LL, Argani P et al (2007) Molecular Definition of Breast Tumor Heterogeneity. Cancer Cell 11:259–273. https://doi.org/10.1016/j.ccr.2007.01.013
Junttila MR, de Sauvage FJ (2013) Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501:346–354. https://doi.org/10.1038/nature12626
Schmitt MW, Loeb LA, Salk JJ (2016) The influence of subclonal resistance mutations on targeted cancer therapy. Nat Rev Clin Oncol 13:335–347. https://doi.org/10.1038/nrclinonc.2015.175
Lee HY, Jeong JY, Lee KS et al (2012) Solitary pulmonary nodular lung adenocarcinoma: correlation of histopathologic scoring and patient survival with imaging biomarkers. Radiology 264:884–893. https://doi.org/10.1148/radiol.12111793
Lee G, Lee HY, Park H et al (2017) Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: State of the art. Eur J Radiol 86:297–307. https://doi.org/10.1016/j.ejrad.2016.09.005
Ciernik IF, Dizendorf E, Baumert BG et al (2003) Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol 57:853–863. https://doi.org/10.1016/S0360-3016(03)00346-8
Fischer B, Lassen U, Mortensen J et al (2009) Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 361:32–39. https://doi.org/10.1056/NEJMoa0900043
Ohri N, Duan F, MacHtay M et al (2015) Pretreatment FDG-PET metrics in stage III non-small cell lung cancer: ACRIN 6668/RTOG 0235. J Natl Cancer Inst 107:1–7. https://doi.org/10.1093/jnci/djv004
Lewis DY, Soloviev D, Brindle KM (2015) Imaging tumor metabolism using positron emission tomography. Cancer J 21:129–136. https://doi.org/10.1097/PPO.0000000000000105
Gümüştaş S, Inan N, Akansel G et al (2012) Differentiation of malignant and benign lung lesions with diffusion-weighted MR imaging. Radiol Oncol 46:106–113. https://doi.org/10.2478/v10019-012-0021-3
Sagiyama K, Watanabe Y, Kamei R et al (2017) Multiparametric voxel-based analyses of standardised uptake values and apparent diffusion coefficients of soft-tissue tumours with a positron emission tomography/magnetic resonance system: Preliminary results. Eur Radiol 27:5024–5033. https://doi.org/10.1007/s00330-017-4912-y
Yoon HJ, Kim Y, Kim BS (2015) Intratumoral metabolic heterogeneity predicts invasive components in breast ductal carcinoma in situ. Eur Radiol 25:3648–3658. https://doi.org/10.1007/s00330-015-3761-9
Lee HY, Jeong JY, Lee KS et al (2013) Histopathology of lung adenocarcinoma based on new IASLC/ATS/ERS classification: Prognostic stratification with functional and metabolic imaging biomarkers. J Magn Reson Imaging 38:905–913. https://doi.org/10.1002/jmri.24080
Wu J, Cui Y, Sun X et al (2017) Unsupervised clustering of Quantitative image phenotypes reveals breast cancer subtypes with distinct prognoses and Molecular pathways. Clin Cancer Res 23:3334–3342. https://doi.org/10.1158/1078-0432.CCR-16-2415 Personalized
Inano R, Oishi N, Kunieda T et al (2016) Visualization of heterogeneity and regional grading of gliomas by multiple features using magnetic resonance-based clustered images. Sci Rep 6:30344. https://doi.org/10.1038/srep30344
Metz S, Ganter C, Lorenzen S et al (2015) Multiparametric MR and PET imaging of intratumoral biological heterogeneity in patients with metastatic lung cancer using voxel-by-voxel analysis. PLoS One 10:1–14. https://doi.org/10.1371/journal.pone.0132386
Gabow H (2007) Proceedings of the Eighteenth Annual ACM-SIAM Symposium on Discrete Algorithms. Society for Industrial and Applied Mathematics, Philadelphia, PA, USA
Levine E, Domany E (2001) Resampling Method for Unsupervised Estimation of Cluster Validity. Neural Comput 13:2573–2593. https://doi.org/10.1162/089976601753196030
Paesmans M, Berghmans T, Dusart M et al (2010) Primary tumor standardised uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: Update of a systematic review and meta-analysis by the european lung cancer working part. J Thorac Oncol 5:612–619. https://doi.org/10.1097/JTO.0b013e3181d0a4f5
Ng S-H, Yen T-C, JT-C C et al (2006) Prospective Study of [18 F]Fluorodeoxyglucose Positron Emission Tomography and Computed Tomography and Magnetic Resonance Imaging in Oral Cavity Squamous Cell Carcinoma With Palpably Negative Neck. J Clin Oncol 24:4371–4376. https://doi.org/10.1200/JCO.2006.05.7349
Simon RM, Subramanian J, Li MC, Menezes S (2011) Using cross-validation to evaluate predictive accuracy of survival risk classifiers based on high-dimensional data. Brief Bioinform 12:203–214. https://doi.org/10.1093/bib/bbr001
Anderson K, Lutz C, van Delft FW et al (2011) Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 469:356–361. https://doi.org/10.1038/nature09650
Landau DA, Carter SL, Stojanov P et al (2013) Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152:714–726. https://doi.org/10.1016/j.cell.2013.01.019
Burrell RA, McGranahan N, Bartek J, Swanton C (2013) The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501:338–345. https://doi.org/10.1038/nature12625
Horswell S, Matthews N, Swanton C (2013) Cancer heterogeneity and “The struggle for existence”: Diagnostic and analytical challenges. Cancer Lett 340:220–226. https://doi.org/10.1016/j.canlet.2012.10.031
Schmitz J, Schwab J, Schwenck J et al (2016) Decoding intratumoral heterogeneity of breast cancer by multiparametric in vivo imaging: A translational study. Cancer Res 76:5512–5522. https://doi.org/10.1158/0008-5472.CAN-15-0642 Tumor
Divine MR, Katiyar P, Kohlhofer U et al (2016) A Population-Based Gaussian Mixture Model Incorporating 18F-FDG PET and Diffusion-Weighted MRI Quantifies Tumor Tissue Classes. J Nucl Med 57:473–479. https://doi.org/10.2967/jnumed.115.163972
Turkki R, Linder N, Holopainen T et al (2015) Assessment of tumour viability in human lung cancer xenografts with texture-based image analysis. J Clin Pathol:1–8. https://doi.org/10.1136/jclinpath-2015-202888
Wu J, Gensheimer MF, Dong X et al (2016) Robust Intratumor Partitioning to Identify High-Risk Subregions in Lung Cancer: A Pilot Study. Int J Radiat Oncol Biol Phys 95:1504–1512. https://doi.org/10.1016/j.ijrobp.2016.03.018
Kozak MM, Murphy JD, Schipper ML et al (2011) Tumor Volume as a Potential Imaging-Based Risk-Stratification Factor in Trimodality Therapy for Locally Advanced Non-small Cell Lung Cancer. J Thorac Oncol 6:920–926. https://doi.org/10.1097/JTO.0b013e31821517db
Henzler T, Goldstraw P, Wenz F et al (2015) Perspectives of Novel Imaging Techniques for Staging, Therapy Response Assessment, and Monitoring of Surveillance in Lung Cancer: Summary of the Dresden 2013 Post WCLC-IASLC State-of-the-Art Imaging Workshop. J Thorac Oncol 10:237–249. https://doi.org/10.1097/JTO.0000000000000412
Lee JH, Lee HY, Ahn M-J et al (2016) Volume-based growth tumor kinetics as a prognostic biomarker for patients with EGFR mutant lung adenocarcinoma undergoing EGFR tyrosine kinase inhibitor therapy: a case control study. Cancer Imaging 16:5. https://doi.org/10.1186/s40644-016-0063-7
Funding
This study was supported by the Institute for Basic Science (grant number IBS-R015-D1), the National Research Foundation of Korea (grant numbers NRF-2016R1A2B4008545, NRF-2016R1A2B4013046, and NRF-2017M2A2A7A02018568), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (grant number HI17C0086), and Guerbet Korea Ltd.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ho Yun Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Kim, J., Ryu, SY., Lee, SH. et al. Clustering approach to identify intratumour heterogeneity combining FDG PET and diffusion-weighted MRI in lung adenocarcinoma. Eur Radiol 29, 468–475 (2019). https://doi.org/10.1007/s00330-018-5590-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5590-0