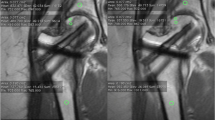
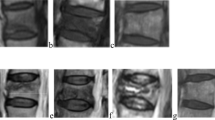
Abstract
Objectives
To prospectively evaluate whether dynamic contrast-enhanced (DCE) MRI can assess vascularity within non-unions and predicts clinical outcome in combination with the clinical Non-Union Scoring System (NUSS).
Methods
Fifty-eight patients with non-unions of extremities on CT underwent 3-T DCE MRI. Signal intensity curves obtained from a region-of-interest analysis were subdivided into those with more intense contrast agent uptake within the non-union than in adjacent muscle (vascularised non-union) and those with similar or less contrast uptake. The pharmacokinetic parameters of the Tofts model K trans, K ep, iAUC and V e were correlated with union at CT 1 year later (n = 49).
Results
Despite inserted osteosynthetic material, DCE parameters could be evaluated in 57 fractures. The sensitivity/specificity of vascularised non-unions as an indicator of good outcome was 83.9 %/50.0 % compared to 96.8 %/33.3 % using NUSS (n = 49). Logistic regression revealed a significant impact of NUSS on outcome (P = 0.04, odds ratio = 0.93). At first examination, median iAUC (initial area under the enhancement curve) for the ratio non-union/muscle was 10.28 in patients with good outcome compared with 3.77 in non-responders (P = 0.023). K trans, K ep and Ve within the non-union were not significantly different initially (n = 57) or 1 year later (n = 19).
Conclusions
DCE MRI can assess vascularity in fracture non-unions. A vascularised non-union correlates with good outcome.
Key points
• Dynamic contrast-enhanced magnetic resonance imaging can assess vascularity within bony non-unions.
• Vascularised ununited fractures appear better at 1-year CT than poorly vascularised fractures.
• Non-union healing after osteosynthesis or osteoinductive drugs fundamentally requires vascularity.
• DCE MRI predicts treatment outcome better than the clinical Non-Union Scoring System.
• DCE MRI is clinically feasible to predict treatment outcome in bony non-unions.





Similar content being viewed by others
Abbreviations
- CEUS:
-
Contrast-enhanced ultrasound
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DCE MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- FOV:
-
Field of view
- iAUC:
-
Initial area under the enhancement curve
- K ep :
-
Constant reflux between extravascular extracellular space and blood plasma
- K trans :
-
Volume transfer constant between blood plasma and extravascular extracellular space
- NUSS:
-
Non-Union Scoring System
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- STIR:
-
Short tau inversion recovery
- TE:
-
Echo time
- TR:
-
Repetition time
- TSE:
-
Turbo-spin-echo
- V e :
-
Extravascular extracellular volume fraction
- VIBE:
-
Volume interpolated breath-hold examination
References
Chapman S (1992) The radiological dating of injuries. Arch Dis Child 67:1063–1065
Cruess RL, Dumont J (1975) Fracture healing. Can J Surg 18:403–413
Frost HM (1989) The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop Relat Res 248:294–309
Hendrex RW (1992) Fracture healing. In: Rogers LF (ed) Radiology of skeletal trauma, 2nd edn. Churchill Livingstone, New York, pp 203–221
Marsh D (1998) Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res 355:S22–S30
Kuhlman JE, Fishman EK, Magid D, Scott WW Jr, Brooker AF, Siegelman SS (1988) Fracture nonunion: CT assessment with multiplanar reconstruction. Radiology 167:483–488
Bhattacharyya T, Bouchard KA, Phadke A, Meigs JB, Kassarjian A, Salamipour H (2006) The accuracy of computed tomography for the diagnosis of tibial nonunion. J Bone Joint Surg Am 88:692–697
Calori GM, Mazza E, Colombo M, Ripamonti C, Tagliabue L (2011) Treatment of long bone non-unions with polytherapy: indications and clinical results. Injury 42:587–590
Calori GM, Tagliabue L, Gala L, d’Imporzano M, Peretti G, Albisetti W (2008) Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury 39:1391–1402
Friedlaender GE, Perry CR, Cole JD et al (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am 83-A:S151–S158
Garrison KR, Shemilt I, Donell S et al (2010) Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev 6, CD006950
Geesink RG, Hoefnagels NH, Bulstra SK (1999) Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br 81:710–718
Giannoudis PV, Einhorn TA, Schmidmaier G, Marsh D (2008) The diamond concept-open questions. Injury 39:S5–S8
Govender S, Csimma C, Genant HK et al (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84-A:2123–2134
Schmidmaier G, Schwabe P, Wildemann B, Haas NP (2007) Use of bone morphogenetic proteins for treatment of non-unions and future perspectives. Injury 38:S35–S41
Schmidmaier G, Capanna R, Wildemann B, Beque T, Lowenberg D (2009) Bone morphogenetic proteins in critical-size bone defects: what are the options? Injury 40:S39–S43
Giannoudis PV, Einhorn TA, Marsh D (2007) Fracture healing: the diamond concept. Injury 38:S3–S6
Tzioupis C, Giannoudis PV (2007) Prevalence of long-bone non-unions. Injury 38:S3–S9
Calori GM, Phillips M, Jeetle S, Tagliabue L, Giannoudis PV (2008) Classification of non-union: need for a new scoring system? Injury 39:S59–S63
Weber BG, Cech O (1976) Pseudoarthrosis: Pathology, biomechanics, therapy, results. Hans Huber, Bern
Calori GM, Giannoudis PV (2011) Enhancement of fracture healing with the diamond concept: the role of the biological chamber. Injury 42:1191–1193
Choyke PL, Dwyer AJ, Knopp MV (2003) Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 17:509–520
Hawighorst H, Libicher M, Knopp MV, Moehler T, Kauffmann GW, Kaick G (1999) Evaluation of angiogenesis and perfusion of bone marrow lesions: role of semiquantitative and quantitative dynamic MRI. J Magn Reson Imaging 10:286–294
Libicher M, Bernd L, Schenk JP, Madler U, Grenacher L, Kauffmann GW (2001) Characteristic perfusion pattern of osseous giant cell tumor in dynamic contrast-enhanced MRI. Radiologe 41:577–582
Libicher M, Kasperk C, Daniels M, Hosch W, Kauczor HU, Delorme S (2008) Dynamic contrast-enhanced MRI in Paget's disease of bone—correlation of regional microcirculation and bone turnover. Eur Radiol 18:1005–1011
Munk PL, Lee MJ, Janzen DL et al (1998) Gadolinium-enhanced dynamic MRI of the fractured carpal scaphoid: preliminary results. Australas Radiol 42:10–15
Hirata T, Konishiike T, Kawai A, Sato T, Inoue H (2001) Dynamic magnetic resonance imaging of femoral head perfusion in femoral neck fracture. Clin Orthop Relat Res 393:294–301
Griffith JF, Yeung DK, Tsang PH et al (2008) Compromised bone marrow perfusion in osteoporosis. J Bone Miner Res 23:1068–1075
Yeung DK, Lam SL, Griffith JF et al (2008) Analysis of bone marrow fatty acid composition using high-resolution proton NMR spectroscopy. Chem Phys Lipids 151:103–109
Dyke JP, Aaron RK (2010) Noninvasive methods of measuring bone blood perfusion. Ann N Y Acad Sci 1192:95–102
Ehrhart N, Kraft S, Conover D, Rosier RN, Schwarz EM (2008) Quantification of massive allograft healing with dynamic contrast enhanced-MRI and cone beam-CT: a pilot study. Clin Orthop Relat Res 466:1897–1904
Klaue K, Knothe U, Anton C et al (2009) Bone regeneration in long-bone defects: tissue compartmentalisation? In vivo study on bone defects in sheep. Injury 40:S95–S102
Masquelet AC, Begue T (2010) The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am 41:27–37
Pelissier P, Martin D, Baudet J, Lepreux S, Masquelet AC (2002) Behaviour of cancellous bone graft placed in induced membranes. Br J Plast Surg 55:596–598
Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J (2004) Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res 22:73–79
Savolaine ER, Ebraheim N (2000) Assessment of femoral neck nonunion with multiplanar computed tomography reconstruction. Orthopedics 23:713–715
Slade JF 3rd, Gillon T (2008) Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg 97:280–289
Taylor JS, Tofts PS, Port R et al (1999) MR imaging of tumor microcirculation: promise for the new millennium. J Magn Reson Imaging 10:903–907
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Keramaris NC, Calori GM, Nikolaou VS et al (2008) Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury 39:S45–S57
Abumunaser LA, Al-Sayyad MJ (2011) Evaluation of the Calori et al nonunion scoring system in a retrospective case series. Orthopedics 34:359. doi:10.3928/01477447-20110317-31
Giannoudis PV, Tzioupis C (2005) Clinical applications of BMP-7: the UK perspective. Injury 36:S47–S50
Weber MA, Krix M, Delorme S (2007) Quantitative evaluation of muscle perfusion with CEUS and with MR. Eur Radiol 17:2663–2674
Slotboom J, Schaer R, Ozdoba C et al (2008) A novel method for analyzing DSCE-images with an application to tumor grading. Invest Radiol 43:843–853
Wetzel SG et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803
Nosàs-Garcia S, Moehler T, Wasser K et al (2005) Dynamic contrast-enhanced MRI for assessing the disease activity of multiple myeloma: a comparative study with histology and clinical markers. J Magn Reson Imaging 22:154–162
Shapeero LG, Poffyn B, De Visschere PJ et al (2011) Complications of bone tumors after multimodal therapy. Eur J Radiol 77:51–67
Jans L, De Coninck T, Wittoek R et al (2013) 3 T DCE-MRI assessment of synovitis of the interphalangeal joints in patients with erosive osteoarthritis for treatment response monitoring. Skeletal Radiol 42:255–260
Krix M, Weber MA, Krakowski-Roosen H et al (2005) Assessment of skeletal muscle perfusion using contrast-enhanced ultrasonography. J Ultrasound Med 24:431–441
Weber MA, Krakowski-Roosen H, Delorme S et al (2006) Relationship of skeletal muscle perfusion measured by contrast-enhanced ultrasonography to histologic microvascular density. J Ultrasound Med 25:583–591
Lang P, Honda G, Roberts T et al (1995) Musculoskeletal neoplasm: perineoplastic edema versus tumor on dynamic postcontrast MR images with spatial mapping of instantaneous enhancement rates. Radiology 197:831–839
Verstraete KL, Lang P (2000) Bone and soft tissue tumors: the role of contrast agents for MR imaging. Eur J Radiol 34:229–246
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schoierer, O., Bloess, K., Bender, D. et al. Dynamic contrast-enhanced magnetic resonance imaging can assess vascularity within fracture non-unions and predicts good outcome. Eur Radiol 24, 449–459 (2014). https://doi.org/10.1007/s00330-013-3043-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-3043-3