Abstract
Despite the general belief that the Southern Ocean harbors low fish biodiversity, the Weddell Sea hosts one of the richest fish communities in the region. Parallelly, the Weddell Sea is also known for the presence of dense and diverse macrobenthos. Most macrobenthic invertebrates, such as gorgonians, sponges and bryozoans, are considered ecosystem engineers as they generate a three-dimensional structure that increases habitat heterogeneity. This structural complexity serves as a refuge against predators as well as a nursery ground for many organisms, including fish species. By analyzing video transects recorded by a Remotely Operated Vehicle, we investigated density, spatial distribution and size-frequency of populations of the demersal fish species inhabiting macrobenthic communities in the southernmost part of the Weddell Sea. We also attempted to unveil whether there is any relationship between benthic and fish communities and substrate, as well as some fish behavioral patterns. The dominance of juveniles in the surveyed fish assemblages provides evidence that, at this life stage, some fish species appear to be positively associated with complex benthic communities conformed by bryozoans, sponges and gorgonians which are more common in sand matrix with sparse rocks substrates. Moreover, about 37% of all specimens recorded were resting on benthic invertebrates or were using them to hide, implying that Antarctic benthic communities might offer suitable habitat. As such, it can be concluded that there was an apparent relationship between certain species of fish and the different benthic communities, yet the exact triggers and/or factors behind such an association remain partially elusive.
Similar content being viewed by others
Introduction
The Southern Ocean is the water body occupying a total area of 34.8 million km2 comprised between the coast of the Antarctic continent and the Antarctic Polar Front (also known as Antarctic Convergence, located at about 50°S in the Atlantic and Indian sectors and 60°S in the Pacific sector) (Hofmann 1985). It is characterized by low water temperature and seasonal primary production due to seasonal ice cover, which has remained practically unaltered over the last 20 million years (Clarke and Crame 1989). Yet, despite its large dimension and long evolutionary history, fish biodiversity is less diverse than expected (Eastman 2005).
Fish species with benthic habits are the most diverse and abundant fish group in Antarctic waters, representing more than 60% of all Antarctic fish species, with the Notothenioidei suborder being the major component in terms of biomass and number of demersal species (DeWitt 1971; Eastman and Hubold 1999; Eastman et al. 2013). In fact, most of the notothenioid species are endemic to the Southern Ocean (Andriashev 1965; Kock 1992). This high endemism has occurred due to the Antarctic Polar Front, which acts as a zoogeographic boundary, as well as the constant and severe conditions of the Southern Ocean waters (Eastman and Clarke 1998; Peck 2018). The Notothenioidei currently includes 9 families and 140 species, from which 5 families and 110 species are exclusively present in Antarctic waters, while only 4 families and 30 species are non-Antarctic (Eastman and Eakin 2021; Daane and Detrich 2022).
Despite the aforementioned generalization that the Southern Ocean presents lower fish diversity than expected, the Weddell Sea stands out as a hotspot of fish diversity within the area, harboring the highest number of fish species within the Southern Ocean (Hubold 1991). Within the High-Antarctic Zone (sensu Kock 1992), the Weddell Sea is characterized by a wide shelf as deep as more than 1000 m (Ekau 1990; Anderson 1999). It also shows the most durable sea-ice coverage, which plays a fundamental role in physical and biological processes, such as the formation of the coldest water masses and productivity (Clarke and Ackley 1984; Peck 2018). Furthermore, the bottom of the High-Antarctic Zone stands out due to the presence of diverse and dense microbenthic communities, known as the Antarctic Marine Animal Forests (AMAF) (Arntz et al. 1994; Gutt et al. 2004; Gili et al. 2006). Most of the macrobenthic sessile organisms in these communities, such as gorgonians, sponges and bryozoans, are considered ecosystem engineers since they generate a three-dimensional structure that increases habitat heterogeneity (Jones et al. 1994; Gili and Coma 1998; Rossi et al. 2017). This increase in structural complexity provides a wide array of niches in the areas those species occur, which in turn might serve as a refuge against predators or as nursery grounds for many other species, including fish species (Gutt et al. 1994; Gratwicke and Speight 2005; Buhl-Mortensen et al. 2010; La Mesa et al. 2019). In this regard, previous studies have noted that some fish species settle on elevated benthic structures such as sponges, a behavior considered characteristic for certain species (Ekau and Gutt 1991; Gutt and Ekau 1996). Yet, while macrobenthic sessile organisms might increase the diversity and density of other taxa, their presence does not always lead to an increase in diversity, as the degree of habitat modification depends on the density and size of the engineer species (Jones et al. 1994; Cerrano et al. 2010).
In the past, it was only possible to study marine ecosystems through destructive methods but, thanks to technological advances, that has changed. Technological advances and the development of remotely operated vehicles (ROVs), manned submersibles, autonomous underwater vehicles (AUVs) and ocean floor observation systems (OFOS) have made it possible to go further in understanding the marine environment, enabling an in situ overview of the ecosystem (Gutt and Ekau 1996; Santín et al. 2018; La Mesa et al. 2022). While catch sampling is a destructive technique that normally results in a disordered sampling of animals, underwater photography and video are conservative methods that significantly minimize impacts on sessile organisms. Image methods allow for direct observation, as well as a much greater study area in terms of extension and depth, compared to using destructive methods (Ninio et al. 2003; Matarrese et al. 2004; Rossi et al. 2008). As so, underwater video and photo technology has been used for studies on biomass (Mühlenhardt-Siegel et al. 1988; Pabis et al. 2011), animal behavior (Ekau and Gutt 1991; La Mesa et al. 2019) demography and spatial distribution of benthic (Segelken-Voigt et al. 2016; Ambroso et al. 2017) and motile species (Ekau and Gutt 1991; Lorance and Trenkel 2006; Amsler et al. 2015; La Mesa et al. 2022). Moreover, some studies have provided the same results on the distribution and abundance of fish species by comparing catch sampling and image techniques, validating the usage of the later for fish community studies (Ekau and Gutt 1991; Gutt et al. 1994; La Mesa et al. 2019). Yet, despite the possibilities offered by imaging techniques, few studies have focused on the macrobenthic species that accompany fish assemblages in different study areas (Gutt et al. 1994; Gutt and Ekau 1996; La Mesa et al. 2019).
Hence, the present study aims to fill the said knowledge gap and respond to the hypothesis of a possible relationship between complex benthic communities and greater biodiversity of Antarctic fishes. In order to achieve this, three main objectives have been pursued through video analyses: (1) identification of demersal fish species, their density, spatial distribution and size-frequency distribution populations; (2) description of fish assemblages; and (3) a study of the relationship between benthic communities and the different fish species, including any behavioral patterns observed.
Material and methods
Study area
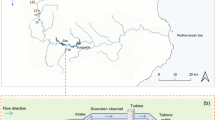
The study area was located in front of the Filchner-Ronne Ice Shelf (FRIS), in the southernmost part of the Weddell Sea (Fig. 1), which has, until now, been poorly studied due to heavy sea-ice conditions and consequently difficult access. Therefore, any new information about this region is of special relevance. This area is characterized by cold and dense water masses with a cyclonic clockwise flow (Deacon 1979; Daae et al. 2020). Near the bottom, the Weddell Sea Bottom Water (WSBW) can be found, which is slightly warmer than the Weddell Sea Deep Water (WSDW). Above this, there is the Warm Deep Water (WDW), which occupies the layer of 200–1200 m depth and its temperature is between − 1 and – 2 °C (CTD data). In addition to this, the continental shelf includes two water masses that make up the Dense Shelf Water (DSW): The Ice Shelf Water (ISW) and the High-Salinity Shelf Water (HSSW). They originate from the sea-ice production (Haid and Timmermann 2013) and, in turn, contribute to the formation of the Weddell Sea Deep and Bottom Waters (Gordon et al. 1993; Orsi et al. 1999). All of the conditions of the shelf water masses have remained practically stable since the 1980s (Janout et al. 2021). The entire area is mainly characterized by soft bottoms with different proportions of sand, gravel and boulders (Anderson et al. 1980; Haase 1986).
Video recording
A total of six video transects were analyzed, covering a total area of 1,845.9 m2 between 251 and 361 m depth, as part of the multidisciplinary scientific cruise PS82 (ANT XXIX/9) expedition on board the RV Polarstern, conducted from December 2013 to March 2014.
To study the composition and distribution of Antarctic fishes and their relationships with invertebrate benthic macrofauna, an inspection-class ROV (Ocean Modules V8Sii) was deployed at six stations in the area of the Filchner Trough (Fig. 1). The videos were acquired with a high definition (HD) video camera (Kongsberg oe14–502) recording with a viewing angle of 29° × 45° (vertical × horizontal) and equipped with two lasers with a 4 cm separation that serves as a reference scale, used for the spatial density and fish size analyses. Furthermore, the ROV was equipped with ultra-short baseline (USBL) positioning data. All of the ROV video material pertaining to this study is available from the data publisher PANGAEA® at www.pangaea.de (Owsianowski et al. 2017).
Video analysis
Video transects were analyzed using Premiere Pro CC version 12.1.2 (Adobe Inc.) software and following the methodology described in Gori et al. (2011). Each track was edited before the analysis in order to remove pauses, loops or video sequences where the image quality was not suitable for the study, for example, when there was sediment resuspension. After editing, every benthic organism and fish observed along the transect was annotated, within a section of 0.3 m width around the central position of the laser beams. A reference time was assigned to each benthic organism and fish was when it was observed and their position along the transect was recorded with geographical coordinates. In addition, following the same analysis procedure, substrate type and depth was annotated along each transect. Substrate types were classified into three categories, namely: sand matrix with rocks, gravel matrix, and rock matrix. Seabed slope was also analyzed, and it was consistently found to be 0.
Data analysis
Once fish species and benthic organisms had been identified and georeferenced, it was decided to take an area of 50 m2 as a sampling unit (0.3 m width and 166.5 m length) to analyze the data statically. Since the target fish of the study are demersal, but the benthic communities are mainly composed of sessile animals and could present a highly patchy distribution, the sampling unit chosen to study both of them is 50 m2. Each sampling unit was characterized by a type of macrobenthic community and substrate. The slope of all the transects was zero, and the sea bottom was horizontal. This factor was therefore not considered to characterize the sampling units, and not used for statistical analysis either. Depth was also ruled out as an influencing factor in this study because an ADONIS test was carried out using the vegan package (Oksanen et al. 2013) from RStudio (RStudio Team 2019), and no influence of depth was found on the studied fish assemblages. The study area corresponds to a unique water mass (WDW), and that could be the reason why depth did not appear to be an influent factor in this case. Regarding benthic classification, four benthic communities were defined by the most abundant engineer species: bryozoan community (B), when more than 85% of the macrobenthic organisms were bryozoans and no other macrobenthic phylum reached 10% coverage by itself; bryozoan and sponge community (BS), when bryozoan represented more than 60% and sponges more than 15% of the macrobenthos; sponge and bryozoan community (SB), which was dominated by sponges, with any inverse distribution (≥ 60% and ≥ 15%, respectively); and lastly, bryozoan, gorgonian and sponge community (BGS), where bryozoans represented more than 60%, gorgonians more than 20%, and sponges more than 10% (Fig. 2). Besides the types of benthic communities, two kinds of bottom substrate were defined: sand matrix with sparse rocks and gravel matrix. Each sampling unit usually presented a combination of both, but was defined by the most abundant (with a percentage of 80% or more).
Density and spatial distribution and size-frequency of fish populations
For statistical analysis, all of the transects were divided into sampling units of 50 m2 (0.3 m width and 166.5 m length). A total of 37 sampling units were obtained from six transects, composed of four sampling units of transect 49, four more of transect 81, eight of transect 86, seven of transect 128, seven sampling units of transect 136, and seven more of transect 170, respectively. In order to study the distribution for the most representative species, a geographically-referenced map was generated using QGIS version 3.16 (QGIS.org 2022), in which both fish density and the corresponding type of benthic community of the sampling unit were represented. In addition, the species composition was examined and their density (ind. 100 m−2) were quantified.
The size-frequency of fish populations was computed only for those species with more than 13 individuals documented. The ROV lasers were set at a known distance from each other (30 cm) and provided a scale for the measurement of fish individuals. When a fish appeared in the video transect, a photo was taken when the lasers were parallel to the individual, which was then analyzed for measurements by means of the software Macnification version 2.0.5 (Schols and Lorson 2008) In order to compare species with different size population, size-frequency histograms were performed for each species by means of the ggplot2 package (Wickham 2016) from the R software platform (RStudio Team 2019). In the High Antarctic, more than 90% of notothenioids do not reach 45 cm total length, and other fishes like artedidraconids are typically less than 15 cm. The variability of maximum fish sizes between observed fish species is the reason why the sizes of fishes are classified in small ranges of 3 cm.
Fish assemblage composition
Aiming to find some assemblage present in the study area, abundances of fish species were represented by means of a dendrogram and non-metric multi-dimensional scaling (nMDS). Before the analysis, the abundance data were transformed using the square-root method to normalize them, and then distances between pairs of sampling units were calculated using the Bray–Curtis dissimilarity index. To obtain the dendrogram, the hclust function of vegan package (Oksanen et al. 2013) was used from the RStudio platform (RStudio Team 2019). In order to elaborate the nMDS, the metaMDS function was applied, again available via the vegan package (Oksanen et al. 2013). Three sampling units were discarded from the statistical analysis, having no fish because of the analysis requirements. Dendrograms and nMDS are only representations of how sampling units are grouped, as an exploratory method to graphically visualize if there might be some kind of relationship among the data. The ADONIS test was applied using the vegan package (Oksanen et al. 2013) to determine any statistical differences among fish assemblages with RStudio software (RStudio Team 2019). To carry out these analyses, individuals classified at the genus or family level have not been considered, because they could be different species and, therefore, could have different substrate and benthic community preferences, which could introduce errors in the interpretation of the results.
Relationship with environmental features
The distribution of fish species, like all other animals, is determined by environmental features. In this sense, in the present study, the environmental factors determining fish distribution were explored by canonical correspondence analysis (CCA), which is a multivariate analysis. CCA allows the identification of probable relationships between fish species and environmental factors (Greenacre 2013). The environmental features considered were depth, substrate, and benthic community. Sampling units without fish were excluded because of the statistical test requirements. For this reason, only a total of 34 sampling units were analyzed. Additionally, the individuals not classified at species level were deleted for this analysis, for the same reason as in the dendrogram analysis. Another statistical test to find out how fish species are related to environmental factors was Indicator Value Analysis (IndVal), which was described by Dufrêne and Legendre (1997). The analysis presents a table of indicator species that are quite abundant and significantly characteristic in a type of habitat and with a p-value of less than 0.05. In this study, this was tested with the different types of benthic communities and substrates previously defined, using the indval function of the labdsv package (Roberts and Roberts 2016) of RStudio (RStudio Team 2019). In addition, the richness and diversity of fish species were quantified by means of the Shannon Diversity Index, which was calculated for the different types of benthic communities and substrates. This is represented in two box plots carried out via the ggplot package (Wickham 2016) of RStudio (RStudio Team 2019).
Results
Density, spatial distribution and size-frequency of fish populations
A total of six stations of video transects of the seabed were conducted in the Weddell Sea from 251 to 361 m water depth, covering an area of 1.845,9 m2. Thanks to the high resolution of the camera, the majority of the fishes observed were identified at species level. The rest of them were identified within a family or genus category. In these cases, the high swimming speed or the incomplete vision of the body prevent identification with the highest level. Overall, 12 fish genera and 14 fish species were identified. A total of 414 specimens were recorded that could be identified to species level (87.2%), and the rest of the individuals have been identified to genus (11.3%) and family level (1.4%). Icefish of the genus Chionodraco were not identified to the species level, as we were not able to clearly distinguish between them. The major diversity and density of fish were found at stations 128, 136 and 170 (Tables 1 and 2). Fishes recorded belonged to four families, such as Artedidraconidae, Bathydraconidae, Channichthyidae and Nototheniidae. The family Nototheniidae was the most abundant, including in decreasing order of abundance Trematomus scotti (Boulenger 1907) (30.7%), T. lepidorhinus (Pappenheim 1911) (13.8%), and T. eulepidotus (Regan 1914) (10.4%). The Channichtyidae was the second most abundant and speciose family, encompassing at least six different species, followed by the Bathydraconidae with four species, whereas the Artedidraconidae family was represented by a single specimen of Pogonophryne scotti (Regan 1914). In terms of spatial distribution, only the six most abundant species were represented in maps (Fig. 60). Chionodraco spp. and Chaenodraco wilsoni (Regan 1914) were observed only in northern stations, while T.scotti, T. eulepidotus, T. lepidorhinus and Pagetopsis maculatus (Barsukov and Permitin 1958) were distributed both in the northern and southern stations. The size-frequency distributions are summarized in Fig. 3. The size population of the fish species was described only when the number of observations of each species amounted to more than 13 individuals (Fig. 4). All nototheniids belonged to Trematomus genus and reached a maximum total length (TL) of 21 cm, with a modal size of 9 cm. This pattern of size distribution can also be observed with the specimens of Pagetopsis, whereas Cryodraco antarcticus (Dollo 1900) and Chionodraco spp. showed the largest sizes reaching more than 30 cm TL. In Chionodraco spp. it should be highlighted the absence of individuals smaller than 18 cm TL.
Fish assemblage composition
To find out the fish assemblage composition, a dendrogram was performed representing the sample clustering based on a Bray–Curtis dissimilarity matrix (Fig. 5). Cluster analysis separated five assemblages (at 60% of dissimilarity), with the 8, 9 and 13 sampling units being considered as outliers. Most of the sample units classified as BGS benthic community (red color in the cluster, Fig. 5) are grouped in the representation of the cluster, and all of them are characterized by sand as the most abundant substrate. The second group contains all the SB sampling units (grey color, Fig. 5), and the majority of them correspond to the matrix of gravel substrate. The last group shows the majority of the BS sampling units (green color, Fig. 5) and this grouping can be divided into two depending on the substrate. As a result, fish distribution appears to be related benthic communities and substrate.
Dendrogram representing the sampling units clustering based on a Bray–Curtis dissimilarity. The colors of the sampling units indicate the type of benthic community. Orange bryozoan community. Green bryozoan and sponge community. Red bryozoan, gorgonian and sponge community. Grey sponge and bryozoan community. The asterisk indicates sampling units with gravel bottom and the absence of it indicates sampling units defined as sand matrix with rocks
The non-metric multi-dimensional scaling (nMDS) was another analysis to pursue some relation between benthic communities, substrate and fish distribution (Fig. 6). The ordination of the sampling units that correspond to the SB community are clearly grouped; the same occurs with the BGS community, and some grouping formed by the sampling units of the BS community could also be seen. Consequently, there could be a correlation between fish biodiversity and density and these types of benthic communities. On the other hand, despite some sampling units of the B community also being grouped, others are not. Regarding the substrate, there is a spatial ordination of the different types of substrate that proves that there also seems to be a relationship between this environmental factor and the distribution and abundance of fish species. All this coincides with the result obtained from the dendrogram. In addition, the ADONIS test confirmed that benthic communities are different (p-value < 0.01) (Table 3). Comparing benthic communities in pairs, results show that all of them were different from each other, except for the B and BS communities (p-value = 0.238) (Table 3). The two categories of substrate, sand matrix with some rocks and gravel matrix, also appear to be different from each other (p-value < 0.001).
NMDS biplot of Bray–Curtis dissimilarity matrix of the different sampling units analyzed. Each sample unit was classified with a type of benthic community and substrate, as well as the abundances of the observed fish species were studied. Orange bryozoan community. Green bryozoan and sponge community. Red bryozoan, gorgonian and sponge community. Grey Sponge and bryozoan community. Triangle gravel substrate. Circle sand matrix with rocks
Relationship with environmental features
Regarding geographical distribution and benthic communities' preferences of the studied fish species (Fig. 3; Table 4), P. scotti and Gymnodraco acuticeps (Boulenger 1902) were observed exclusively restricted to the BS community, whereas Pagetopsis spp. and Trematomus loennbergii (Regan 1913) were recorded within the BGS community. Gerlachea australis (Dollo 1900), Racovitzia glacialis (Dollo 1900), Chionodraco spp., C. antarcticus, Prionodraco evansii (Regan, 1914) and some non-identified individuals belonging to the Channichthyidae family were associated to the BS and BGS communities. Pagetopsis macropterus (Boulenger 1907) and C. wilsoni occurred within three benthic communities B, BS and BGS, whereas the remaining species were observed everywhere. The highest densities were found within the BS community, where T. scotti density was 8 ind. m−2, and also in the BGS community, where the maximum density of C. wilsoni was 6.75 ind. m−2, but the latter was the benthic community with the highest total density of fish species. All species were observed on sampling units with a matrix of sand with rocks, yet some species were also found on sampling units defined as having gravel bottoms, but with low densities, such as P. maculatus and P. macropterus. Conversely, T. lepidorhinus is the only species that showed higher density on gravel bottoms, yet it occurred on both types of substrate.
As observed in the results obtained with the dendrogram and nMDS, it seems that fish assemblage has a relation with benthic communities and substrate. To delve further into this, a CCA was performed that shows the relations between fish species with the benthic community and substrate (Fig. 7). Sand substrate with rocks seems to have a strong relationship with the vast majority of identified species, except for T. lepidorhinus. Regarding the benthic communities, most fish species are positively related to BGS communities, such as G. australis, T. loennbergii, C. wilsoni and C. antarcticus. Differently, G. acuticeps and P. scotti prefer the BS community, and Cygnograco mawsoni (Waite 1916) seems to be more associated with B community. The CCA analysis is complemented by a statistic analysis, the Indicator Value Analysis (IndVal), which shows, with a p-value of less than 0.05, that T. lepidorrhinus is characteristic of the SB community, Chionodraco spp. are common in the BS community, and the C. wilsoni, C. antarcticus, T. scotti, G. australis and T. loennbergii species are characteristic in the BGS community. Moreover, this same test shows, with a p-value of < 0.05, that T. lepidorrhinus is typical on gravel bottoms, while T. scotti, T. eulepidotus, P. maculatus and C. wilsoni are more characteristic on sandy ground.
In agreement with our previous results, Shannon’s index was highest in BGS community and lowest in SB community (Fig. 8). Regarding substrate, sand matrix with sparse rocks shows the highest Shannon values, so this type of substrate appears to favor fish biodiversity and abundance. In addition, it should be said that the most complex benthic communities studied (BGS) were observed only in sampling units with sand matrix with rocks, so a relationship might also be deduced. Conversely, in sampling units with a gravel matrix, simpler benthic communities have been observed. In fact, most of them were B communities. Indeed, the only three fishless sampling units that were excluded from the dendrogram and CCA analyses are predominantly characterized by a gravel substrate and B community. Therefore, the results seem to be consistent. The biodiversity indexes were calculated considering all the fishes identified at species and genus level. Following this, one genus could be represented by more than one species, so the biodiversity index calculated could be the same or less than real biodiversity index.
Discussion
The analysis of the images has allowed the identification of a total of four fish families: Artedidraconidae, Bathydraconidae, Channichthyidae and Nototheniidae of the perciform suborder Notothenioidei. These families are the most abundant in the High-Antarctic Zone, especially on the continental shelf of the Weddell Sea (Ekau 1990). Nevertheless, other families occur in the High-Antarctic Zone, like Liparididae and Zoarcidae, which are less abundant but equally diverse as those mentioned, were not observed in this survey (Anderson 1990). In this sense, some fishes may be so elusive to the presence of submersible devices, to the point of becoming undetectable (Katsanevakis et al. 2012). As a result of this circumstance, the number of observations in this study could be underestimated. Throughout the study area, the fish assemblage was mainly characterized by representatives of the family Nototheniidae, being Trematomus the most abundant genus, followed by the Channichthyidae family, whose most representative genera were Pagetopsis and Chionodraco (Table 2), in accordance with data from previous studies in the southeastern Weddell Sea and other regions of the High-Antarctic Zone (DeWitt 1971; Ekau 1990; La Mesa et al. 2019). This contrasts with the Antarctic Peninsula that belongs to Low-Antarctic Zone also called Seasonal Pack-Ice Zone, where the Nototheniops spp. and Notothenia spp. were predominant (Targett 1981; Kock and Stransky 2000).
Matching the size-frequency distributions of fishes with size (Fig. 4) at sexual maturity reported elsewhere, it was possible to infer the population structure of the species recorded. Among nototheniids, T. eulepidotus and T. lepidorhinus attain sexual maturity at 21–24 and 18–21 cm TL, respectively (DeWitt et al. 1990; Duhamel et al. 1993; La Mesa et al. 2008), so the population surveyed consisted exclusively of juveniles and subadults (Fig. 4). Conversely, the population of T. scotti was composed of both juveniles and adults, as they reach sexual maturity at 12–13 cm TL (Duhamel et al. 1993). In this sense, although belonging to the same genus, different species might present contrasting population demographics, highlighting the importance of accurate identification of the specimens. Among channichthyids, P. macropterus and P. maculatus attain sexual maturity at 18–19 cm TL, whereas for C. wilsoni it is at 23–25 cm TL (Duhamel et al. 1993; Kock et al. 2008), so their populations consisted solely of juveniles and, in the case of C. wilsoni, with the additional presence of just a few adults (Fig. 4). Similarly, all the specimens of C. antarcticus were juveniles, as they reach sexual maturity at a size larger than 35 cm (Kock and Jones 2002), which were not observed in the present study (Fig. 4). These results are consistent with previous studies (La Mesa et al. 2020), confirming that early juveniles show a pelagic distribution and feed on krill, mysids and fish larvae, while adults are demersal and more sedentary, and hunt prey following the "sit and wait" feeding strategy (Ekau and Gutt 1991; Kock et al. 2013). Conversely, the populations of Chionodraco spp. included both juveniles and adults as C. myersi (DeWitt and Tyler 1960) and C. hamatus (Lönnberg, 1905) attain sexual maturity at 25–30 cm and 35 cm TL, respectively) (Duhamel et al. 1993; La Mesa et al. 2003). In summary, T. eulepidotus, T. lepidorhinus, T. scotti, C. wilsoni, P. maculatus and P. macropterus, six of eight species whose size population has been analyzed, exhibited a population dominated by juvenile stages. These species have in common that they reflect some dependence on the water column, since much of their diet is made up of krill or other euphausiids (Targett 1981; La Mesa et al. 2004). These results lead to hypothesize that adult individuals might show more active behavior for prey in the water column, while juveniles belonging to these species might prefer the most complex benthic communities formed by sponges, bryozoans and gorgonians, which provide a suitable place to hide from predators until they reach a larger size.
In this sense, observation in situ by means of video transects also allowed us to assess the relationships between fish and benthic communities, as well as to evaluate their habitat preferences and specific behaviors. As has been mentioned previously, benthic organisms such as sponges, gorgonians and bryozoans offer protection, food and a place for breeding and nursery for different notothenioid fishes (Ekau 1990; Ekau and Gutt 1991). In agreement with previous studies (Ekau and Gutt 1991; Gutt and Ekau 1996; La Mesa et al. 2019) T. lepidorhinus prefers benthic communities characterized by populations of sponges and bryozoans. In fact, during the course of this study T. lepidorhinus had often been observed resting on or hiding inside volcano sponges (more than 60% of observations for this species). Similarly, several species (T. scotti, T. loennbergii, C. antarcticus, C. wilsoni and G. australis) were common where bryozoans, gorgonians and sponges are abundant (Table 4; Fig. 7). Some authors explain this preference by the three-dimensional structure that these engineer animals offer for protection against predators, being an advantage for the "sit-observe and hide" strategy of some fish species during their juvenile stage (Gutt and Ekau 1996; La Mesa et al. 2019). Further, these kinds of complex benthic communities offer suitable habitats for sedentary species that use the same strategy but for hunting. They lie in wait hidden and hunt on the prowl (Ekau and Gutt 1991). Conversely, was C. mawsoni the only fish species which showed a preferential relationship for bryozoan communities and more open areas without high-growing sponges (Table 4; Fig. 7) (Ekau and Gutt 1991). Regarding substrate preference observed, fish diversity rose in sandy areas with rocks where the most complex benthic communities were common and consequently, the variety of ecological niches were greater (Fig. 8). Conversely, fish biodiversity decreased in gravel areas hosting simpler benthic communities (Fig. 8). In this sense, the different kinds of substrates and benthic communities studied make that fish assemblage vary depending on them. Consistent with their benthic and sedentary behaviors, about 37% of all specimens recorded were resting on benthic invertebrates such as sponges and bryozoans or were using the tridimensional structures created by the macrobenthos to hide. The rest of the observed fishes were found swimming a few centimeters above the substrate. It should be also mentioned that C. wilsoni was observed seven times out of 31 sightings guarding eggs on nets composed of flat drop stones, with a behavior previously observed in the west Antarctic Peninsula (Ziegler et al. 2017) and the Weddell Sea (La Mesa et al. 2019; Kock et al. 2008). For this reason, in order to ward off potential predators such as starfish and other fish species, nesting and parental care are common in demersal species like C. wilsoni (Kock et al. 2006).
To conclude, the distribution and abundance of fish species depend on a large number of factors not considered in this research, but which are still important, such as temperature and food availability (Ekau and Gutt 1991). Further, ice-scouring also represents a determining factor in the distribution of species at a small scale in High-Antarctic waters (Brenner et al. 2001). This study proves that seems to be a close relationship between the different species of fish observed and the different benthic communities defined by the predominant engineer organisms, and consequently, by their structural complexity. Fish species take advantage of the structures of benthic organisms to hide from predators, like T. lepidorhinus, or to hunt on the prowl as C. antarcticus does. Despite there not being a clear correlation with all of the fish species, biodiversity is indeed greater in sandy areas with rocks where complex benthic communities are more common.
Data availability
PANGAEA.® at www.pangaea.de. Owsianowski, N., Federwisch, L., Kluibenschedl, A., Casado de Amezua, MP., and Richter, C. (2017): Sea-floor videos (benthos) along 12 ROV profiles during POLARSTERN cruise PS82 (ANT-XXIX/9). Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.879283
References
Ambroso S, Salazar J, Zapata-Guardiola R, Federwisch L, Richter C, Gili JM, Teixidó N (2017) Pristine populations of habitat-forming gorgonian species on the Antarctic continental shelf. Sci Rep 7(1):1–11. https://doi.org/10.1038/s41598-017-12427-y
Amsler MO, Eastman JT, Smith KE, Mcclintock JB, Singh H, Thatje S, Aronson RB (2015) Zonation of demersal fishes off Anvers Island, western Antarctic Peninsula. Antarct Sci 28(1):44–50. https://doi.org/10.1017/S0954102015000462
Anderson JB (1999) Antarctic marine geology. Cambridge University Press, Cambridge
Anderson JB, Kurtz DD, Domack EW, Balshaw KM (1980) Glacial and glacial marine sediments of the Antarctic continental shelf. J Geol 88(4):399–414. https://doi.org/10.1086/628524
Andriashev AP (1965) A general review of the Antarctic fish fauna. Biogeogr Ecol Antarct. https://doi.org/10.1007/978-94-015-7204-0_15
Arntz WE, Brey T, Gallardo VA (1994) Antarctic zoobenthos. In: Ansell AD, Gibson RN, Barnes M (eds) Oceanography and marine biology: an annual review, vol 32. UCL Press, London, pp 241–304
Brenner M, Buck BH, Cordes S et al (2001) The role of iceberg scours in niche separation within the Antarctic fish genus Trematomus. Polar Biol 24:502–507. https://doi.org/10.1007/s003000100246
Buhl-Mortensen L, Vanreusel A, Gooday AJ, Levin LA, Priede IG, Buhl-Mortensen P et al (2010) Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar Ecol 31(1):21–50. https://doi.org/10.1111/j.1439-0485.2010.00359.x
Cerrano C, Danovaro R, Gambi C, Pusceddu A, Riva A, Schiaparelli S (2010) Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodiversity Conserv 19(1):153–167. https://doi.org/10.1007/s10531-009-9712-5
Clarke DB, Ackley SF (1984) Sea ice structure and biological activity in the Antarctic marginal ice zone. J Geophy Res C 89(C2):2087–2095. https://doi.org/10.1029/JC089iC02p02087
Clarke A, Crame JA (1989) The origin of the Southern Ocean marine fauna. Geo Soc Spec Publ 47(1):253–268. https://doi.org/10.1144/GSL.SP.1989.047.01.19
Daae K, Hattermann T, Darelius E, Mueller RD, Naughten KA, Timmermann R, Hellmer HH (2020) Necessary conditions for warm inflow toward the Filchner Ice Shelf, Weddell Sea. Geophys Res Lett. https://doi.org/10.1029/2020GL089237
Daane JM, Detrich HW (2022) Adaptations and diversity of Antarctic fishes: a genomic perspective. Annu Rev Anim Biosci 10:39–62. https://doi.org/10.1146/annurev-animal-081221-064325
Deacon GER (1979) The Weddell Gyre. Deep-Sea Res 26(9):981–995. https://doi.org/10.1016/0198-0149(79)90044-X
DeWitt HH (1971) Coastal and deep-water benthic fishes of the Antarctic. In: Bushnell VC (ed) Antarctic map folio series, Folio 15. AGS, New York, pp 1–10
DeWitt HH, Tyler JC (1960) Fishes of the Stanford Antarctic Biological Research Program, 1958–1959. Stanf Ichthyol Bull 7:162–199
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67(3):345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Duhamel G, Kock KH, Balguerias E, Hureau JC (1993) Reproduction in fish of the Weddell Sea. Polar Biol 13:193–200. https://doi.org/10.1007/BF00238929
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28(2):93–107. https://doi.org/10.1007/s00300-004-0667-4
Eastman JT (2013) Antarctic fish biology: evolution in a unique environment. Academic Press, Washington, DC
Eastman J, Eakin R (2021) Checklist of the species of notothenioid fishes. Antarct Sci 33(3):273–280. https://doi.org/10.1017/S0954102020000632
Eastman J, Hubold G (1999) The fish fauna of the Ross Sea, Antarctica. Antarct Sci 11(3):293–304. https://doi.org/10.1017/S0954102099000383
Eastman JT, Clarke A (1998) A comparison of adaptive radiations of Antarctic fish with those of NonAntarctic fish. In: Fish of Antarctica. Springer, Milano. https://doi.org/10.1007/978-88-470-2157-0_1
Ekau W (1990) Demersal fish fauna of the Weddell Sea, Antarctica. Antarct Sci 2(2):129–137. https://doi.org/10.1017/S0954102090000165
Ekau W, Gutt J (1991) Notothenioid fishes from the Weddell Sea and their habitat, observed by underwater photography and television. In: Proceedings of NIPR Symposium Polar Biology, vol 4, pp 36–49
Gili JM, Coma R (1998) Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol 13(8):316–321. https://doi.org/10.1016/S0169-5347(98)01365-2
Gili JM, Rossi S, Pagès F, Orejas C, Teixidó N, López-González PJ, Arntz WE (2006) A new trophic link between the pelagic and benthic systems on the Antarctic shelf. Mar Ecol Prog Ser 322:43–49
Gordon AL, Huber BA, Hellmer HH, Ffield A (1993) Deep and bottom water of the Weddell Sea’s western rim. Science 262(5130):95–97
Gori A, Rossi S, Linares C, Berganzo E, Orejas C, Dale MR, Gili JM (2011) Size and spatial structure in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, northwestern Mediterranean Sea). Mar Biol 158(8):1721–1732. https://doi.org/10.1007/s00227-011-1686-7
Gratwicke B, Speight RM (2005) Effects of habitat complexity on Caribbean marine fish assemblages. Mar Ecol Prog Ser 292:301–310. https://doi.org/10.3354/meps292301
Greenacre M (2013) Fuzzy coding in constrained ordinations. Ecology 94(2):280–286. https://doi.org/10.1890/12-0981.1
Gutt J, Ekau W (1996) Habitat partitioning of dominant high Antarctic demersal fish in the Weddell Sea and Lazarev Sea. J Exp Mar Biol Ecol 206(1–2):25–37. https://doi.org/10.1016/0022-0981(95)00186-7
Gutt J, Sirenko BI, Smirnov IS, Arntz WE (2004) How many macrozoobenthic species might inhabit the Antarctic shelf ? Antarct Sci 16(1):11–16. https://doi.org/10.1017/S0954102004001750
Gutt J, Ekau W, Gorny M (1994) New results on the fish and shrimp fauna of the Weddell Sea and Lazarev Sea (Antarctic). In: Proceedings of NIPR Symposium on Polar Biology, vol 7, pp 91–102
Haase GM (1986) Glaciomarine sediments along the Filchner/Rønne Ice Shelf, southern Weddell Sea—first results of the 1983/84 Antarktis-II/4 expedition. Mar Geol 72(3–4):241–258. https://doi.org/10.1016/0025-3227(86)90122-2
Haid V, Timmermann R (2013) Simulated heat flux and sea ice production at coastal polynyas in the southwestern Weddell Sea. J Geophys Res C 118:2640–2652. https://doi.org/10.1002/jgrc.20133
Hofmann EE (1985) The large-scale horizontal structure of the Antarctic circumpolar current from FGGE drifters. J Geophys Res 90:7087–7097. https://doi.org/10.1029/jc090ic04p07087
Hubold G (1991) Ecology of Notothenioid Fish in the Weddell Sea. Biol Antarct Fish. https://doi.org/10.1007/978-3-642-76217-8_1
Janout MA, Hellmer HH, Hattermann T, Huhn O, Sültenfuss J, Østerhus S et al (2021) FRIS revisited in 2018: on the circulation and water masses at the Filchner and Ronne Ice Shelves in the southern Weddell Sea. J Geophys Res C. https://doi.org/10.1029/2021JC017269
Jones CG, Lawton JH, Shachak M, Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers organisms as ecosystem engineers. Oikos 69(3):373–386
Katsanevakis S, Weber A, Pipitone C, Leopold M, Cronin M, Scheidat M et al (2012) Monitoring marine populations and communities: methods dealing with imperfect detectability. Aquat Biol 16(1):31–52. https://doi.org/10.3354/ab00426
Kock KH (1992) Antarctic fish and fisheries. Cambridge University Press, Cambridge
Kock KH, Jones CD (2002) The biology of the icefish Cryodraco antarcticus Dollo, 1900 (Pisces, Channichthyidae) in the southern Scotia Arc (Antarctica). Polar Biol 25(6):416–424. https://doi.org/10.1007/s00300-002-0357-z
Kock KH, Stransky C (2000) The composition of the coastal fish fauna around Elephant Island (South Shetland Islands, Antarctica). Polar Biol 23(12):825–832. https://doi.org/10.1007/s003000000159
Kock K, Pshenichnov L, Devries A (2006) Evidence for egg brooding and parental care in icefish and other notothenioids in the Southern Ocean. Antarct Sci 18(2):223–227. https://doi.org/10.1017/S0954102006000265
Kock KH, Gröger J, Jones CD (2013) Interannual variability in the feeding of ice fish (Notothenioidei, Channichthyidae) in the southern Scotia Arc and the Antarctic Peninsula region (CCAMLR Subareas 48.1 and 48.2). Polar Biol 36(10):1451–1462. https://doi.org/10.1007/s00300-013-1363-z
Kock KH, Pshenichnov L, Jones CD, Gröger J, Riehl R (2008) The biology of the spiny icefish Chaenodraco wilsoni Regan, 1914. Polar Biol 31(3):381–393. https://doi.org/10.1007/s00300-007-0366-z
La Mesa M, Caputo V, Rampa R, Vacchi M (2003) Macroscopic and histological analyses of gonads during the spawning season of Chionodraco hamatus (Pisces, Channichthyidae) off Terra Nova Bay, Ross Sea. Southern Ocean Polar Biol 26(9):621–628. https://doi.org/10.1007/s00300-003-0519-7
La Mesa M, Eastman JT, Vacchi M (2004) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27(6):321–338. https://doi.org/10.1007/s00300-004-0599-z
La Mesa M, Caputo V, Eastman JT (2008) The reproductive biology of two epibenthic species of Antarctic nototheniid fish of the genus Trematomus. Antarct Sci 20(4):355–364. https://doi.org/10.1017/S095410200800103X
La Mesa M, Piepenburg D, Pineda-Metz SE, Riginella E, Eastman JT (2019) Spatial distribution and habitat preferences of demersal fish assemblages in the southeastern Weddell Sea (Southern Ocean). Polar Biol 42(5):1025–1040. https://doi.org/10.1007/s00300-019-02495-3
La Mesa M, Calì F, Riginella E, Mazzoldi C, Jones CD (2020) Biological parameters of the High-Antarctic icefish, Cryodraco antarcticus (Channichthyidae) from the South Shetland Islands. Polar Biol 43(2):143–155. https://doi.org/10.1007/s00300-019-02617-x
La Mesa M, Canese S, Montagna P, Schiaparelli S (2022) Underwater photographic survey of coastal fish community of Terra Nova Bay. Ross Sea Diversity 14(5):315. https://doi.org/10.3390/d14050315
Lorance P, Trenkel VM (2006) Variability in natural behavior, and observed reactions to an ROV, by mid-slope fish species. J Exp Mar Biol Ecol 332(1):106–119. https://doi.org/10.1016/j.jembe.2005.11.007
Matarrese A, Mastrototaro F, D’onghia G, Maiorano P, Tursi A (2004) Mapping of the benthic communities in the Taranto seas using side-scan sonar and an underwater video camera. Chem Ecol 20(5):377–386. https://doi.org/10.1080/02757540410001727981
Mühlenhardt-Siegel U (1988) Some results on quantitative investigations of macrozoobenthos in the Scotia Arct (Antarctica). Polar Biol 8(4):241–248
Ninio R, Delean S, Osborne K, Sweatman H (2003) Estimating cover of benthic organisms from underwater video images: variability associated with multiple observers. Mar Ecol Prog Ser 265:107–116. https://doi.org/10.3354/meps265107
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, Ohara RB et al (2013) Package ‘vegan.’ Community Ecol Pack Version 2(9):1–295
Orsi AH, Johnson GC, Bullister JL (1999) Circulation, mixing, and production of Antarctic Bottom Water. Prog Oceanogr 43(1):55–109. https://doi.org/10.1016/S0079-6611(99)00004-X
Owsianowski N, Federwisch L, Kluibenschedl A, Casado de Amezua MP, Richter C (2017): Sea-floor videos (benthos) along 12 ROV profiles during POLARSTERN cruise PS82 (ANT-XXIX/9). Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.879283
Pabis K, Sicinski J, Krymarys M (2011) Distribution patterns in the biomass of macrozoobenthic communities in Admiralty Bay (King George Island, south Shetlands, Antarctic). Polar Biol 34(4):489–500. https://doi.org/10.1007/s00300-010-0903-z
Peck LS (2018) Antarctic marine biodiversity: adaptations, environments and responses to change. Oceanogr Mar Biol, Annu Rev 56:105–236
QGIS.org (2022) QGIS Geographic Information System. QGIS Association. http://www.qgis.org
Roberts DW, Roberts MDW (2016) Package ‘labdsv’. Ordination and multivariate, vol 775
Rossi S, Tsounis G, Orejas C, Padrón T, Gili JM, Bramanti L et al (2008) Survey of deep-dwelling red coral (Corallium rubrum) populations at Cap de Creus (NW Mediterranean). Mar Biol 154(3):533–545. https://doi.org/10.1007/s00227-008-0947-6
Rossi S, Bramanti L, Gori A, Orejas C (2017) Animal forests of the world: an overview. Mar Anim For. https://doi.org/10.1007/978-3-319-21012-4
RStudio Team (2020) RStudio: integrated development for R. RStudio, PBC, Boston
Santín A, Grinyó J, Ambroso S, Uriz MJ, Gori A, Dominguez-Carrió C, Gili JM (2018) Sponge assemblages on the deep Mediterranean continental shelf and slope (Menorca Channel, Western Mediterranean Sea). Deep Sea Res Part I 131:75–86
Schols P, Lorson D (2008) Macnification. Orbicule, Leuven, Belgium
Segelken-Voigt A, Bracher A, Dorschel B, Gutt J, Huneke W, Link H, Piepenburg D (2016) Spatial distribution patterns of ascidians (Ascidiacea: Tunicata) on the continental shelves off the northern Antarctic Peninsula. Polar Biol 39(5):863–879. https://doi.org/10.1007/s00300-016-1909-y
Targett TE (1981) Trophic ecology and structure of coastal Antarctic fish communities. Mar Ecol Prog Ser 4:243–263
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer, New York. ISBN 978-3-319-24277-4. https://ggplot2.tidyverse.org
Ziegler AF, Smith CR, Edwards KF, Vernet M (2017) Glacial dropstones: islands enhancing seafloor species richness of benthic megafauna in West Antarctic Peninsula fjords. Mar Ecol Prog Ser 583:1–14. https://doi.org/10.3354/meps12363
Acknowledgements
We thank to the captain and crew of R/V Polarstern cruise PS82 (ANT-XXIX/9) and AWI for their technical and logistical support. This research was accomplished based on the Polarstern Grant No: AWI_PS82_03 and partially funded by ECOWED Project (CTM2012-39350-C02-01), PACES I 1.6, PACES II 1.6. We are grateful to Anna Kluibenschedl, Santiago Pineda Metz and Pilar Casado de Amezua for their help during ROV deployments. Financial support is also acknowledged to Fundación Biodiversidad, MitiCap and ResCap projects through the contracts to Stefano Ambroso, Andreu Santín and Patricia Baena. In addition, the authors affiliated to the Institut de Ciències del Mar had the institutional support of the “Severo Ochoa Centre of Excellence” accreditation (CEX2019-000928-S).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
PB: Wrote the main manuscript text, analyzed the videos and did the statistical analyses AS: Support in the approach of the manuscript, identification of benthic species and statistical analyses MM and ER : Identification of fish species NO: Main pilot of ROV JMG: Identification of benthic species SA: Support in the approach of the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors of this study declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baena, P., Santín, A., La Mesa, M. et al. Are there distribution patterns and population structure differences among demersal fish species in relation to Antarctic benthic communities? A case study in the Weddell Sea. Polar Biol 46, 1069–1082 (2023). https://doi.org/10.1007/s00300-023-03184-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03184-y











