Abstract
Seabirds often spend time on the water in the vicinity of their breeding colonies at the start or end of foraging trips, which may be for bathing, social interaction, information transfer, or to reduce predation risk for small petrels that prefer to return to land in darkness. Although such behaviour (hereafter rafting) is common, there are few data on variation in its incidence or timing across species, or analyses of relationships with intrinsic or extrinsic factors such as breeding stage (reflecting central-place foraging constraints) or weather. Here, we use GPS and immersion data collected over multiple years at Bird Island, South Georgia, to investigate rafting behaviour of four albatross and one burrow-nesting petrel species. Nearly all tracked birds (89%) landed within 10 km of the colony at the start of foraging trips for ~ 30 min, whereas only 17% did so at the end, suggesting they likely use rafting mainly for plumage maintenance after extended breeding shifts on land. Rafting duration, distance and bearing from the colony varied markedly according to species, wind speeds and period of the day (daylight vs. darkness), which may reflect differences in foraging direction, time constraints, degree of plumage soiling, diel activity patterns, or the requirement for high wind speeds for efficient flight. Given that all the study populations are decreasing, and most individuals make extensive use of nearshore waters during the breeding season, effective marine spatial planning is required that eliminates or mitigates human risks around their colonies.
Similar content being viewed by others
Introduction
Many seabird species are known to spend time on the sea surface in the vicinity of their breeding colonies either at the start or end of foraging trips. This behaviour is usually considered to serve one or more of the following functions: bathing, social interaction, information transfer, or reducing predation risk (Wilson et al. 2009; Weimerskirch et al. 2010; Carter et al. 2016; Granadeiro et al. 2018; Richards et al. 2019). As seabirds often breed in dense colonies where their plumage can become soiled from nest or burrow maintenance, guano from neighbours, windblown particles, etc., bathing before the commute to foraging grounds allows individuals to clean their feathers, maintaining waterproofing and improving flight efficiency (Granadeiro et al. 2018; Sánchez-Román et al. 2019). Birds may have been at the colony for days without access to water, and so may also land on the sea straight after departure to drink and rehydrate (Weimerskirch et al. 2010; Granadeiro et al. 2018). According to the Information Centre Hypothesis (Ward and Zahavi 1973), aggregations of rafting birds may serve as sources of social information, helping individuals to find mates (Daniels et al. 1994), make decisions about where to recruit (Halley et al. 1995), or signal the direction of profitable patches of prey (Weimerskirch et al. 2010).
Rafting off the colony at the end of the foraging trip is likely to serve different functions. According to the Selfish Herd Principle (Hamilton 1971), temporary animal aggregations which are not the consequence of aggregated resources such as food should provide adaptive benefits to individuals. Most small petrels prefer to return to land in darkness to reduce predation risk, and it is logical that a bird which returns close to the colony towards the end of the day should raft there until after twilight has ended. Indeed, Scopoli’s shearwater Calonectris diomedea extend the duration of their incoming rafting bouts to delay return to their burrows until after the moon has set (Rubolini et al. 2015). Regardless of whether it is daylight or darkness, rafting would also be adaptive if it improves coordination of return onto land by waves of individuals, leading to swamping of predators or kleptoparasites (Le Corre and Jouventin 1997; Wilson et al. 2008).
As a very large proportion of birds from a seabird population may engage in rafting (Granadeiro et al. 2018), waters close to the colony may be of high conservation value and require stringent management regimes to reduce anthropogenic risks. Seabirds are among the most threatened groups of birds due to their extreme life histories, colonial breeding habits and extensive at-sea distributions, all of which expose them to numerous hazards, including invasive alien species, incidental mortality (bycatch) in fisheries, climate change and disease (Dias et al. 2019; Phillips et al. 2022). Tracking of seabirds has been key to identifying areas of intensive use at sea that require protection (Le Corre et al. 2012; Lascelles et al. 2016; Hays et al. 2019; Beal et al. 2021). However, these efforts have largely focussed on detecting where seabirds forage, which can be at considerable distances from colonies, even during the breeding season (Frankish et al. 2020a; Fayet et al. 2021; Soanes et al. 2021). Determining the proportion of a population that rafts, where and for how long, is thus a priority for identifying key areas for birds, and for informing spatial planning such as the implementation of marine extensions to Specially Protected Areas (Wilson et al. 2009).
In this study, we investigated rafting behaviour in four surface-nesting albatrosses and one large, burrow-nesting petrel species breeding at South Georgia: wandering albatross (Diomedea exulans), black-browed albatross (Thalassarche melanophris), grey-headed albatross (Thalassarche chrysostoma), light-mantled albatross (Phoebetria palpebrata) and white-chinned petrel (Procellaria aequinoctialis). This island group supports globally important populations of all these species (12 – 50% of breeding pairs, worldwide; Phillips et al. 2016). All these populations have large foraging ranges during the breeding and non-breeding seasons, and have undergone major declines since the 1980s largely because of bycatch in fisheries, and to a lesser extent oceanographic change (Berrow et al. 2000; Pardo et al. 2017; Poncet et al. 2017). To date, rafting behaviour in these species has only been characterised in black-browed albatrosses at the Falkland Islands (Granadeiro et al. 2018). However, albatrosses, petrels and other seabirds often show extensive variability in their at-sea behaviour according to intrinsic and extrinsic variables (for a review see Phillips et al. 2017). We used GPS and immersion data from tracking of breeding birds from 2008 to 2019 to identify the first and last periods spent on the sea surface during foraging trips, and determine how the characteristics (duration, distance and bearing from the colony) of these rafting periods varied with species, breeding stage, sex, year and time of day. In addition, as these species are reliant on wind for efficient flight (Weimerskirch et al. 2000; Wakefield et al. 2009; Clay et al. 2020), we investigated potential effects of wind speeds on the duration of rafting bouts, hypothesising that low wind speeds, i.e. sub-optimal flight conditions, lead birds to raft for longer.
Methods
Data collection
Fieldwork was carried out at Bird Island, South Georgia (54°00′S, 38°03′W) on wandering albatrosses (January–March 2012, March–April 2015 and March 2019), black-browed albatrosses (January–March 2008, January 2010, December 2014–January 2015 and January 2019), grey-headed albatrosses (December 2009–January 2010), light-mantled albatrosses (December 2009–January 2010 and December 2014 – January 2015) and white-chinned petrels (December 2014–January 2015). Typically, 8–40 birds in each breeding stage (incubation, brood-guard or post-guard chick-rearing) and year were fitted with a GPS logger (i-gotU GT-120, Mobile Action Technology Inc., New Taipei City, Taiwan, or MiniGPSlog or MicroGPSlog, earth & Ocean Technology, Kiel, Germany), attached with Tesa ® tape to mantle feathers, and a combined Global Location Sensor (GLS)-immersion logger (Intigeo C250; Migrate Technology Lt, Cambridge, UK) fitted with cable-ties to a plastic ring on the tarsus. The GPS loggers were programmed with a 5–30-min sampling regime, and the GLS-immersion loggers tested for saltwater immersion every 3 s and recorded the time of transition between wet/dry states that lasted ≥ 6 s. Attachment of devices took < 12 min. Instrument loads (0.2–2.0% of body mass) were well below the threshold where deleterious effects might be expected, and there was no indication that mean foraging trip durations or chick meal mass was affected (Phillips et al. 2003, 2005). For further details see Wakefield et al. 2012, Scales et al. 2016, Clay et al. 2019, 2020; Frankish et al. 2020a, b.
Foraging trip departure and arrival times were estimated to be halfway between the time of the first or last GPS fix at sea, and the previous or subsequent GPS fix at the colony, as appropriate. Occasionally, an adult in the brood-guard or post-guard periods would feed the chick, depart, then return and feed again within the course of a few hours. During this time, the bird was probably close to the colony but not actively foraging, and we therefore only considered absences of > 6 h as foraging trips (following Weimerskirch et al. 1997; Phillips et al. 2003). GPS data were run with an iterative forward/backward averaging filter to remove any locations which required sustained flight speeds above 90 km.h−1 (McConnell et al. 1992), and then linearly interpolated to 1-s intervals and matched with the immersion data to determine where birds had landed on the water surface.
Identification of rafting behaviour
To characterise potential rafting behaviour, we extracted the timings and locations of the first and last major landing event to occur during the outgoing and incoming portion of individual foraging trips. Following Edwards et al. (2007), a landing event (or wet bout) was considered to start when an immersion logger was wet for > 30 s (to exclude instances when the leg might have been immersed briefly during flight) and to end before a dry period that lasted > 30 s (to exclude brief flights). The duration, distance and bearing from the colony of the first and last wet bouts were calculated for every foraging trip. Bearing was calculated using function ‘earth.bear’ in R package ‘fossil’ (Vavrek and Vavrek 2020). Every bout was also assigned from the start time to daylight (‘day’) or darkness (‘night’) using function ‘crepuscule’ in R package ‘maptools’ package to determine the timing of civil twilight (when the sun is 6 degrees below the horizon; Bivand and Lewis-Koh 2017).
Outliers in terms of distance and duration of wet bouts from the colony were removed from the dataset if they had a z-score > 3 (Benhadi-Marín 2018). Five tracks which had incomplete GPS data because of battery or other device failure were also removed. Some birds were tracked for multiple trips, and so to avoid pseudoreplication a single trip was selected at random using the ‘slice_sample’ function in R package ‘dplyr’ (Wickham and Muller 2018). Although there was some variation in GPS sampling interval, degrading the fixes collected more frequently to a 30-min interval made no significant difference to the mean values for distance, duration and bearing of wet bouts relative to the original dataset, suggesting that GPS sampling resolution had minimal effect on our results (see section S1 in Online Resource 1).
To determine whether wind conditions influenced the durations of first and last wet bouts, wind speeds were computed from hourly zonal and meridional wind speed components downloaded from the European Centre for Medium Range Weather Forecasts (ECMWF) ERA5 reanalysis dataset (< https://doi.org/10.24381/cds. adbb2d47 > ; accessed June 2020). This variable was available at a 0.25° spatial resolution, corresponding to around 15–25 km at the latitudes used by tracked birds, and was projected using a Lambert azimuthal equal-area projection centred at 90°S and 38°W. Covariate values were then extracted at each wet bout location.
Statistical analysis
Durations and distances of first and last wet bouts were non-normal in distribution (Shapiro–Wilk normality tests: n = 258 – 265, 0.12 < W < 0.69, all p < 0.0001), and therefore non-parametric statistics were used to investigate differences in these variables between species, daylight vs. darkness, breeding stages (within species), sex and year. A paired Wilcoxon signed-rank test was used to determine whether the duration and distance to Bird Island of first wet bouts differed from those of last wet bouts within individual foraging trips, and a Moore’s test for paired circular data was used to compare the mean bearings (Moore 1980). Those analyses were only conducted for birds for which characteristics of first and last wet bouts were available (n = 252). Kruskal–Wallis non-parametric analysis of variance tests, followed by post hoc multiple comparison tests were used to test for effects of daylight vs. darkness, species, breeding stage (within species), sex and year on the durations and distances of first and last wet bouts from land, and Watson’s non-parametric homogeneity of means test was used to test for effects of these variables on bout bearings (Pewsey et al. 2013). As sample sizes for the comparison of wet bouts in daylight vs. darkness were low (see Table 1), an equal number in each group per species was selected at random using function ‘slice_sample’(Wickham and Muller 2018), and then data were pooled across species for statistical analysis. Differences in rafting characteristics among species were investigated using data from the incubation and brood-guard periods as post-guard data were only available for black-browed albatrosses. Effects of breeding stage were investigated in the three species for which sufficient data were available (black-browed, light-mantled and wandering albatrosses). Effects of sex were tested for species-stage combinations with n ≥ 5 for each sex, and effects of year were investigated using tracking data from black-browed albatrosses during the brood-guard stage, as this was the only species-stage combination with > 2 years of data (2008, 2010, 2015 and 2019).
Linear models were used to determine whether wind speed affected the durations of first and last wet bouts. Species was included as a predictor variable, as was the two-way interaction between wind speed and species, and wet bout duration was log transformed to improve data spread. Model selection was carried out by ranking all possible combinations of predictors according to Akaike Information Criterion (AIC), where the most supported model(s) were considered to be those within 2Δ AIC of the top model (Burnham & Anderson, 2004). Candidate models were excluded from this set if there were simpler nested versions with lower ΔAIC values (Arnold, 2010).
All data processing and analyses were carried out in R, version 4.1.1 (www.r-project.org).
Results
Characteristics of rafting behaviour
Overall, 89% of the first wet bouts (100% for white-chinned petrels and 63—94% for other species) occurred within 10 km of Bird Island [range: 5.3 ± 0.9 to 11.3 ± 49.2 km, depending on species and breeding stage] (Table 1 and Figs. 1b and 2a), i.e. the great majority of tracked birds of all species landed on the water within close range of the colony on the outgoing portion of their foraging trips. By comparison, the average distances from the colony of final wet bouts on the return portion of foraging trips were considerably longer, more variable across species and more dispersed in terms of location; only 26% (34% of black-browed albatrosses and 8–18% of other species) of last wet bouts occurred within 10 km of Bird Island [range: 45.0 ± 73.4 to 182.3 ± 149.7 km, depending on species and breeding stage] (Table 1 and Figs. 1b and 2b). The durations of first and last landings also varied greatly among species (range: 10.7 ± 8.5 to 38.5 ± 32.8 min vs. 8.3 ± 9.8 and 90.2 ± 163.8 min; Table 1), and mostly occurred during daylight (only 10 and 14% of first and last wet bouts, respectively, were in darkness). White-chinned petrels were more likely than the albatross species to depart and return from their foraging trips during darkness (Fig. S1) and 67% of their outgoing wet bouts occurred during darkness (Fig. S2). Only wandering albatrosses conducted final wet bouts during darkness (11% of last wet bouts; Fig. S2), and then usually returned to the colony in the following daylight period (67% of foraging trips).
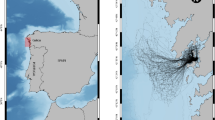
a Location of the study site: Bird Island, South Georgia. The yellow rectangle shows the location and extent of plots shown in the lower panes. b Location of first and last landings (wet bouts) during the foraging trips of seabirds tracked between 2008 and 2019 during the incubation (‘INC’), brood-guard (‘BR’) and post-guard (‘PB’) breeding stages. BBA black-browed albatross (Thalassarche melanophris), GHA grey-headed albatross (Thalassarche chrysostoma), LMA light-mantled albatross (Phoebetria palpebrata), WA wandering albatross (Diomedea exulans) and WCP white-chinned petrel (Procellaria aequinoctialis)
Density plots of distance between Bird Island, South Georgia, and a the first wet bouts, and b the last wet bouts during the foraging trips of seabirds tracked using GPS between 2008 and 2019 during the incubation (‘INC’), brood-guard (‘BR’) and post-guard (‘PB’) breeding stages. BBA black-browed albatross (Thalassarche melanophris), GHA grey-headed albatross (Thalassarche chrysostoma), LMA light-mantled sooty albatross (Phoebetria palpebrata), WA wandering albatross (Diomedea exulans) and WCP white-chinned petrel (Procellaria aequinoctialis)
Differences in rafting behaviour at the start and end of foraging trips were also compared within individuals (Table 1). Compared to the first wet bouts, the last wet bouts were significantly longer in duration (Wilcoxon paired signed-rank test: n = 252, V = 19,297, p = 0.0037), further from the colony (Wilcoxon paired signed-rank test: n = 252, V = 1960, p < 0.0001), and more to the northwest (Moore’s test: n = 252, t = 3.84, p = 0.0001).
Diurnal differences in rafting behaviour
There were no differences between daylight and darkness in the duration (Kruskal–Wallis test: n = 44, H = 0.23, p = 0.6304), distance (Kruskal–Wallis test: n= 44, H = 1.92, p = 0.1661) and bearing from the colony (Watson’s non-parametric homogeneity of means test: n = 44, p = 0.5889) of the first wet bouts on foraging trips. However, on the return, wet bouts during darkness were significantly longer (Kruskal–Wallis test: n = 74, H = 18.28, p < 0.0001) and further from the colony (Kruskal–Wallis test: n= 74, H = 10.48, p = 0.0012), but did not differ in mean bearings (Watson’s non-parametric homogeneity of means test: n = 74, p = 0.1243) from those in daylight.
Inter-specific variation in rafting behaviour
There were significant differences between species in the mean distances and durations of their first (distance: Kruskal–Wallis test, n = 239, H = 22.76, p < 0.0001 and duration: Kruskal–Wallis test, n= 239, H = 29.41, p < 0.0001) and last (distance: Kruskal–Wallis test, n = 233, H = 31.72, p < 0.0001 and duration: Kruskal–Wallis test, n= 233, H = 40.53, p < 0.0001) wet bouts from the colony. Based on multiple comparison tests, on the outgoing portions of trips, light-mantled albatrosses landed significantly further from the colony than black-browed and wandering albatrosses (Figs. 1b and 3a) but spent significantly less time on the water than any other species (Fig. 3b). On the return portion, light-mantled albatrosses landed significantly further from the colony than black-browed and wandering albatrosses (Figs. 1b and 3c), and wandering albatrosses spent significantly longer on the water than black-browed and light-mantled albatrosses, and white-chinned petrels (Fig. 3d). Differences between species in bearing from Bird Island were only significant for the first wet bout (Watson’s non-parametric homogeneity of means test: n = 239, p = 0.0165; Fig. 3e). Grey-headed and wandering albatrosses landed predominantly to the west (~ 270°), black-browed and light-mantled albatrosses slightly more south (~ 265 and ~ 230°, respectively) and white-chinned petrels slightly more north (~ 275°).
Boxplots showing (a) distances between Bird Island, South Georgia, and the first wet bouts conducted during the foraging trips of seabirds tracked from Bird Island (South Georgia) using GPS between 2008 and 2019 during the incubation (‘INC’) and brood-guard (‘BR’) breeding stages. b Species-specific durations of the same wet bouts, c distances and durations of the last wet bouts conducted by the same birds and e density plot of bearings of the first wet bouts. BBA black-browed albatross (Thalassarche melanophris), GHA grey-headed albatross (Thalassarche chrysostoma), LMA light-mantled sooty albatross (Phoebetria palpebrata), WA wandering albatross (Diomedea exulans) and WCP white-chinned petrel (Procellaria aequinoctialis)
Effects of wind on rafting behaviour
The top supported model included the additive effects of hourly wind speeds and species as predictor variables on the durations of first and last wet bouts during foraging trips (Table 2); mean bout durations were longer for all species when wind speeds were lower (Fig. 4).
Predicted linear effect of wind speed and species on the durations of the first (FIRST) and last (LAST) wet bouts conducted during the foraging trips of seabirds tracked from Bird Island, South Georgia, using GPS between 2008 and 2019 during the incubation (‘INC’), brood-guard (‘BR’) and post-guard (‘PB’) breeding stages. Lines and shading represent linear model predictions and 95% confidence intervals, respectively, while faded points represent observed data. Values of transformed response variables are back-transformed on the y-axis but the scale of the transformation (log) is retained. BBA black-browed albatross (Thalassarche melanophris), GHA grey-headed albatross (Thalassarche chrysostoma), LMA light-mantled sooty albatross (Phoebetria palpebrata), WA wandering albatross (Diomedea exulans) and WCP white-chinned petrel (Procellaria aequinoctialis)
Effects of breeding stage on rafting behaviour
There were no significant differences between breeding stages in the duration (Kruskal–Wallis tests, 19 < = n < = 101, 0.14 < = H < = 2.49, all p > = 0.1149) distance (Kruskal–Wallis tests, 19 < = n < = 101, 0.38 < = H < = 1.69, all p > = 0.1936) and bearing (Watson’s non-parametric homogeneity of means tests: 19 < = n < = 101, all p > = 0.0846) of the first and last wet bouts from Bird Island of black-browed, wandering and light-mantled albatrosses. In the only species for which data were available for all three stages (black-browed albatross), there were significant differences in the distances (Kruskal–Wallis test: n = 127, H = 27.42, p < 0.0001) and durations (Kruskal–Wallis test: n = 127, H = 21.94, p < 0.0001) of their first wet bouts, and the bearings of their last wet bouts from the colony (Watson’s non-parametric homogeneity of means tests, n = 127, p = 0.0142). During the post-guard stage, black-browed albatrosses landed significantly closer to Bird Island (n= 127, p < 0.05) and spent significantly longer on the water (n= 127, p < 0.05) than during the incubation and brood-guard stages. Finally, the last wet bout during incubation, brood-guard and post-guard were northwest, directly west and southwest of the colony, respectively. These directions closely resemble directions taken to forage, rather than wind directions upon return to Bird Island (Fig. S3).
Sex differences in rafting behaviour
There were no significant differences between sexes in either distance from the colony (Kruskal–Wallis tests—first: n = 24—65, 0.01 < H < 1.67, all p > = 0.1959; last: n = 24 – 64, 0.01 < H < 1.22, all p > = 0.2700) or duration of first and last wet bouts on foraging trips (Kruskal–Wallis tests—first: n = 24—65, 0.01 < H < 0.89, all p > = 0.3450; last: n = 24 – 64, 0.01 < H < 3.06, all p > = 0.0802). Nor did bearings differ between sexes (Watson’s non-parametric homogeneity of means test—first: n = 24 – 65, all p > 0.1190; last: n = 24 – 64, all p > 0.07), except for wandering albatrosses during incubation (n = 34, p = 0.0067) in which the males landed to the northwest on the outgoing portion, and females were more varied in bearings.
Effects of year on rafting behaviour
In black-browed albatrosses during brood-guard, the durations of first and last wet bouts (Kruskal–Wallis test – first: n = 73, H = 10.35, p = 0.0158, last: n = 72, H = 10.51, p = 0.0147), and distances of first wet bouts only (Kruskal–Wallis tests – first: n = 73, H = 8.86, p = 0.0313) differed significantly among years. The first wet bout was longer in 2008 than in 2015 and 2019 (n = 73, p < 0.05), whereas the last wet bout was longer in 2010 than 2008 (n = 72, p < 0.05). The distance of the first wet bouts in 2019 was slightly longer than in 2008 (n = 73, p < 0.05). Bearings of the first and last wet bouts did not show any significant variation among years (Watson’s non-parametric homogeneity of means tests, n = 72 – 73, all p > = 0.0540).
Discussion
Incidence of rafting at Bird Island, South Georgia
Although many seabirds raft at high densities in waters adjacent to their colonies, this behaviour has been studied in relatively few species (Wilson et al. 2009; Weimerskirch et al. 2010; Carter et al. 2016; Granadeiro et al. 2018; Richards et al. 2019). In our study, although there was marked variation among individuals in the distance from the colony, duration and location of landings, most of the tracked birds (63 – 100% by species; 89% overall) rafted within 10 km of the colony at the start of the foraging trip, and remained on the water for average periods of 30.2 ± 46.5 min (n = 265; 10.7 – 38.5 min, depending on the species). Although these birds were not observed visually, this behaviour is consistent with that of black-browed albatrosses from the Falkland Islands, which were observed bathing within 5 km of the colony for broadly comparable periods (mean 49.5 ± 40.8 min; Granadeiro et al. 2011). Incubation and brooding shifts on land last days to weeks in these species, and hence there are obvious advantages to preening before a foraging trip, when the birds will fly hundreds of km (Clay et al. 2019; Frankish et al. 2020a). In contrast, far fewer returning birds (6 – 34% by species; 17% overall) landed on the water within 10 km of the colony at the end of the foraging trip, and the duration and location of these nearshore landings were very variable (3 – 95.9 min by species, 37 ± 97.2 min overall [n = 258]). In line with other studies, rafting is therefore less important shortly before birds return to land (Carter et al. 2016; Granadeiro et al. 2018). This suggests that the function of rafting in these species is mainly for plumage maintenance. Visual observations close to Bird Island indicated that wandering albatrosses, but not other species, frequently display on the water with wings extended and also call to conspecifics (pers. obs.), indicating that rafting in this species can also serve a secondary, social function. Of our study species, only adult white-chinned petrels are susceptible to predation at the colony (by skuas). As only one of the twelve tracked white-chinned petrels landed on the water near the colony at the end of their foraging trips, they do not appear to use rafting to coordinate return to land as expected if the main function is to reduce predation risk.
As birds in rafts were not observed systematically, we could not test definitively if our study species make use of rafts as information centres, like the Guanay cormorants Phalacrocorax bougainvillii studied by Weimerskirch et al. (2010). However, we do not consider it particularly likely because the albatrosses and white-chinned petrels feed so far from the colony that prey patches are unlikely to persist for long enough to make it profitable for a departing bird to seek the same foraging location as other individuals that are returning. This is corroborated by the lack of site fidelity in black-browed albatrosses, and low fidelity in grey-headed albatrosses at South Georgia over multiple successive foraging trips (Bonnet-Lebrun et al. 2018). Although some black-browed albatrosses were more likely to return to the same foraging area after a more profitable trip (in terms of meal mass fed to the chick), the bearing at departure was not consistent (Bonnet-Lebrun et al. 2021). Hence, it seems that species with long foraging ranges (e.g. > 1000 km during incubation, requiring a minimum of 1–2 days of travel time; Table 1) do not make use of rafting to acquire foraging information (Carter et al. 2016, this study).
Intrinsic and extrinsic drivers of variation in rafting behaviour
Some of the variation in rafting behaviour at the start and end of foraging trips was explained by species, breeding stage and sex. The study species show varying degrees of spatial segregation of their foraging areas, indicative of niche partitioning as a result of competition or habitat specialisation (Phillips et al. 2004, 2005; Clay et al. 2016; Frankish et al. 2020a). Variation among breeding stages in the bearings to the first landings of the tracked black-browed albatrosses may therefore reflect differing directions taken to forage (Frankish et al. 2020a). However, sex differences in bearings to the first landing were generally minor, despite sexual segregation in foraging destinations in several of these species (Phillips et al. 2004; Froy et al. 2015; Pereira et al. 2018).
Other factors, such as nesting on the surface vs. in a burrow may affect the degree of plumage soiling while on land, and could explain why white-chinned petrels spent the most time on the water after leaving the colony. Time pressure may also play a role, as light-mantled albatrosses during incubation make longer trips on average than the other study species (Phillips et al. 2004, 2005, 2006), and they landed furthest away from the colony and for the shortest amount of time. However, time constraints cannot be the only factor as black-browed albatrosses made longer and closer first landings during the post-guard period, which is the most energetically demanding stage of the breeding season (Phillips et al. 2017).
Weather conditions (particularly wind) at departure and return, and the bearing to the feeding area may also influence whether an individual bird lands, where and for how long. Indeed, external conditions are well known to influence various aspects of the behaviour of pelagic seabirds (Phalan et al. 2007; Wakefield et al. 2011; Clay et al. 2020). In our study, the last landings on foraging trips were longer in duration and further from Bird Island during darkness than daylight, which may reflect known diel variation in activity patterns; albatrosses spend more time in flight during daylight, and sit on the sea surface during darkness, either resting or foraging using a ‘sit-and-wait’ strategy (Phalan et al. 2007). Additionally, some albatrosses are observed waiting for dawn to return to the colony (Buller’s albatross, Thalassarche bulleri; Stahl and Sagar 2000), as was mostly the case for wandering albatrosses in this study. Moon phase also influences patterns of colony attendance (Phalan et al. 2007; Rubolini et al. 2015). This may be most relevant for white-chinned petrels, which are very active during darkness but may await safer conditions (i.e. full darkness) before returning to their burrows, to avoid predation by skuas (Berrow and Croxall 1999; Mougeot and Bretagnolle 2000; Rubolini et al. 2015).
In addition, the study species rely heavily on wind for flight (Weimerskirch et al. 2000; Wakefield et al. 2009; Clay et al. 2020). That would explain why we found that all species spent longer rafting during times of reduced windspeed, presumably awaiting better conditions to resume flight away from, or towards the colony. In Manx shearwaters Puffinus puffinus, wind speed increased the size of rafts but wind direction had no influence on rafting location (Richards et al. 2019). Indeed, the location might instead be driven by surface currents, as observed for streaked shearwaters Calonectris leucomelas and Scopoli’s shearwaters C. diomedea (Yoda et al. 2014; Sánchez-Román et al. 2019). However, more research is required to determine how rafting behaviour is affected by environmental conditions and how these might change in the future.
Implications for conservation
Despite marked variation in the timing of rafting, the majority of birds of all five species rafted after departure, and many before return, in waters within a 10 km radius of the colony. This was highly consistent across a large number of study years (2008–2019). There is a large marine-protected area around South Georgia, and regulations are in place that reduce the potential threats from shipping by a ban on use and carriage of heavy fuel oil, and which prohibit fishing within 30 km of land around the mainland and adjacent island, and within 12 nm of Shag and Clerke Rocks (Tancell et al. 2016; Handley et al. 2020). Similar zones elsewhere will almost certainly be important for rafting for all populations of these species, yet coastal waters in general are potentially susceptible to a wide range of anthropogenic threats such as oil spills, disturbance, ship traffic, commercial and recreational fishing (Marcella et al. 2017; Clay et al. 2019). As globally, many populations of albatrosses and large petrels are in decline (Phillips et al. 2016), marine spatial planning and management around seabird colonies clearly has to eliminate or mitigate human risks, for example, by prohibiting fishing or routing shipping beyond rafting distance to protect threatened birds, as well as other marine animals which likely use these areas for essential activities (Handley et al. 2020).
Data availability
The datasets supporting the conclusions of this article are available for download from the BirdLife International Seabird Tracking Database (http://seabirdtracking.org/mapper/contributor.php?contributor_id=361); dataset ids: 1382, 1383, 1384, 1386, 1387, 1537, 2004 and 2005.
Change history
16 June 2023
The supplementary file has been updated
References
Beal M, Dias MP, Phillips RA et al (2021) Global political responsibility for the conservation of albatrosses and large petrels. Sci Adv. https://doi.org/10.1126/sciadv.abd7225
Benhadi-Marín J (2018) A conceptual framework to deal with outliers in ecology. Biodivers Conserv 27:3295–3300. https://doi.org/10.1007/s10531-018-1602-2
Berrow SD, Croxall JP (1999) The diet of white-chinned petrels Procellaria aequinoctialis, Linnaeus 1758, in years of contrasting prey availability at South Georgia. Antarct Sci 11:283–292. https://doi.org/10.1017/S0954102099000371
Berrow SD, Croxall JP, Grant SD (2000) Status of white-chinned petrels Procellaria aequinoctialis Linnaeus 1758, at Bird Island, South Georgia. Antarct Sci 12:399–405. https://doi.org/10.1017/S0954102000000468
Bivand R, Lewis-Koh N (2017) maptools: Tools for Reading and Handling Spatial Objects. R package version 0.9–2. https://CRAN.R-project.org/package=maptools
Bonnet-Lebrun A-S, Phillips RA, Manica A, Rodrigues ASL (2018) Quantifying individual specialization using tracking data: a case study on two species of albatrosses. Mar Biol 165:152. https://doi.org/10.1007/s00227-018-3408-x
Bonnet-Lebrun A-S, Collet J, Phillips RA (2021) A test of the win-stay–lose-shift foraging strategy and its adaptive value in albatrosses. Anim Behav 182:145–151. https://doi.org/10.1016/j.anbehav.2021.10.010
Carter MID, Cox SL, Scales KL et al (2016) GPS tracking reveals rafting behaviour of Northern Gannets (Morus bassanus): implications for foraging ecology and conservation. Bird Study 63:83–95. https://doi.org/10.1080/00063657.2015.1134441
Clay TA, Manica A, Ryan PG et al (2016) Proximate drivers of spatial segregation in non-breeding albatrosses. Sci Rep 6:1–13. https://doi.org/10.1038/srep29932
Clay TA, Small C, Tuck GN et al (2019) A comprehensive large-scale assessment of fisheries bycatch risk to threatened seabird populations. J Appl Ecol 56:1882–1893. https://doi.org/10.1111/1365-2664.13407
Clay TA, Joo R, Weimerskirch H et al (2020) Sex-specific effects of wind on the flight decisions of a sexually dimorphic soaring bird. J Anim Ecol 89:1811–1823. https://doi.org/10.1111/1365-2656.13267
Daniels D, Heath J, Stevanage S (1994) Courtship behaviour in offshore Kittiwake Rissa tridactyla flocks prior to the breeding season. Seabird 16:2–21
Dias MP, Martin R, Pearmain EJ et al (2019) Threats to seabirds: A global assessment. Biol Cons 237:525–537. https://doi.org/10.1016/j.biocon.2019.06.033
Edwards AM, Phillips RA, Watkins NW et al (2007) Revisiting Lévy flight search patterns of wandering albatrosses, bumblebees and deer. Nature 449:1044–1048. https://doi.org/10.1038/nature06199
Fayet AL, Clucas GV, Anker-Nilssen T et al (2021) Local prey shortages drive foraging costs and breeding success in a declining seabird, the Atlantic puffin. J Anim Ecol 90:1152–1164. https://doi.org/10.1111/1365-2656.13442
Frankish CK, Manica A, Phillips RA (2020a) Effects of age on foraging behavior in two closely related albatross species. Mov Ecol 8:7. https://doi.org/10.1186/s40462-020-0194-0
Frankish CK, Phillips RA, Clay TA et al (2020b) Environmental drivers of movement in a threatened seabird: insights from a mechanistic model and implications for conservation. Divers Distrib 26:1315–1329. https://doi.org/10.1111/ddi.13130
Froy H, Lewis S, Catry P et al (2015) Age-related variation in foraging behaviour in the wandering albatross at South Georgia: no evidence for senescence. PLoS ONE. https://doi.org/10.1371/journal.pone.0116415
Granadeiro JP, Brickle P, Catry P (2011) Do individual seabirds specialize in fisheries’ waste? The case of black-browed albatrosses foraging over the Patagonian Shelf. Anim Conserv 17:19–26. https://doi.org/10.1111/acv.12050
Granadeiro JP, Campioni L, Catry P (2018) Albatrosses bathe before departing on a foraging trip: implications for risk assessments and marine spatial planning. Bird Conserv Int 28:208–215. https://doi.org/10.1017/S0959270916000459
Halley DJ, Harris MP, Wanless S (1995) Colony attendance patterns and recruitment in immature Common Murres (Uria aalge). Auk 112:947–957
Hamilton WD (1971) Geometry for the selfish herd. J Theor Biol 31:295–311. https://doi.org/10.1016/0022-5193(71)90189-5
Handley JM, Pearmain EJ, Oppel S et al (2020) Evaluating the effectiveness of a large multi-use MPA in protecting Key Biodiversity Areas for marine predators. Divers Distrib 26:715–729. https://doi.org/10.1111/ddi.13041
Hays GC, Bailey H, Bograd SJ et al (2019) Translating marine animal tracking data into conservation policy and management. Trends Ecol Evol 34:459–473. https://doi.org/10.1016/j.tree.2019.01.009
Lascelles BG, Taylor PR, Miller MGR et al (2016) Applying global criteria to tracking data to define important areas for marine conservation. Divers Distrib 4:422–431. https://doi.org/10.1111/ddi.12411
Le Corre M, Jouventin P (1997) Kleptoparasitism in tropical seabirds: vulnerability and avoidance responses of a host species, the red-footed booby. The Condor 99:162–168. https://doi.org/10.2307/1370234
Le Corre M, Jaeger A, Pinet P et al (2012) Tracking seabirds to identify potential Marine Protected Areas in the tropical western Indian Ocean. Biol Cons 156:83–93. https://doi.org/10.1016/j.biocon.2011.11.015
Marcella TK, Gende SM, Roby DD, Allignol A (2017) Disturbance of a rare seabird by ship-based tourism in a marine protected area. PLoS ONE. https://doi.org/10.1371/journal.pone.0176176
McConnell BJ, Chambers C, Fedak MA (1992) Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarct Sci 4:393–398. https://doi.org/10.1017/S0954102092000580
Moore B (1980) A modification of the rayleigh test for vector data. Biometrika 67:175–180
Mougeot F, Bretagnolle V (2000) Predation risk and moonlight avoidance in nocturnal seabirds. J Avian Biol 31:376–386. https://doi.org/10.1034/j.1600-048X.2000.310314.x
Pardo D, Forcada J, Wood AG, Tuck GN, Ireland L, Pradel R, Croxall JP, Phillips RA (2017) Additive effects of climate and fisheries drive catastrophic declines in multiple albatross species. Proc Nat Acad Sci Unites Stat Amer 114:E10829–E10837
Pereira JM, Paiva VH, Phillips RA, Xavier JC (2018) The devil is in the detail: small-scale sexual segregation despite large-scale spatial overlap in the wandering albatross. Mar Biol 165:55. https://doi.org/10.1007/s00227-018-3316-0
Pewsey A, Neuhauser M, Ruxton G (2013) Circular statistics in R. OUP Oxford.
Phalan B, Phillips RA, Silk J et al (2007) Foraging behaviour of four albatross species by night and day. Mar Ecol Prog Ser 340:271–286. https://doi.org/10.3354/meps340271
Phillips RA, Xavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090. https://doi.org/10.1093/auk/120.4.1082
Phillips RA, Silk JRD, Phalan B et al (2004) Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc Biol Sci 271:1283–1291. https://doi.org/10.1098/rspb.2004.2718
Phillips R, Silk J, Croxall J (2005) Foraging and provisioning strategies of the light-mantled sooty albatross at South Georgia: competition and co-existence with sympatric pelagic predators. Mar Ecol Prog Ser 285:259–270. https://doi.org/10.3354/meps285259
Phillips RA, Silk JRD, Croxall JP, Afanasyev V (2006) Year-round distribution of White-chinned petrels from South Georgia: Relationships with oceanography and fisheries. Biol Cons 129:336–347. https://doi.org/10.1016/j.biocon.2005.10.046
Phillips RA, Gales R, Baker GB et al (2016) The conservation status and priorities for albatrosses and large petrels. Biol Cons 201:169–183. https://doi.org/10.1016/j.biocon.2016.06.017
Phillips RA, Lewis S, González-Solís J, Daunt F (2017) Causes and consequences of individual variability and specialization in foraging and migration strategies of seabirds. Mar Ecol Prog Ser 578:117–150. https://doi.org/10.3354/meps12217
Phillips RA, Fort J, Dias MP (2022) Chapter 2. Conservation status and overview of threats to seabirds. In: Young, L.C. and VanderWerf, E.A. (eds). Conservation of Marine Birds. Elsevier Press. 3–56
Poncet S, Wolfaardt AC, Black A, Browning S, Lawton K, Lee J, Passfield K, Strange G, Phillips RA (2017) Recent trends in numbers of wandering (Diomedea exulans), black-browed (Thalassarche melanophris) and grey-headed (T. chrysostoma) albatrosses breeding at South Georgia. Polar Biol 40:1347–1358
Richards C, Padget O, Guilford T, Bates AE (2019) Manx shearwater (Puffinus puffinus) rafting behaviour revealed by GPS tracking and behavioural observations. Peer J. https://doi.org/10.7717/peerj.7863
Rubolini D, Maggini I, Ambrosini R et al (2015) The effect of moonlight on Scopoli’s shearwater Calonectris diomedea colony attendance patterns and noctural foraging: a test of the foraging efficiency hypothesis. Ethology 121:284–299. https://doi.org/10.1111/eth.12338
Sánchez-Román A, Gómez-Navarro L, Fablet R et al (2019) Rafting behaviour of seabirds as a proxy to describe surface ocean currents in the Balearic Sea. Sci Rep 9:17775. https://doi.org/10.1038/s41598-018-36819-w
Scales KL, Miller PI, Ingram SN et al (2016) Identifying predictable foraging habitats for a wide-ranging marine predator using ensemble ecological niche models. Divers Distrib 22:212–224. https://doi.org/10.1111/ddi.12389
Soanes LM, Green JA, Bolton M, et al (2021) Linking foraging and breeding strategies in tropical seabirds. Journal of Avian Biology 52:jav.02670. https://doi.org/10.1111/jav.02670
Stahl JC, Sagar PM (2000) Foraging strategies of southern Buller’s albatrosses Diomedea b. bulleri breeding on The Snares, New Zealand. J R Soc N Z 30:299–318. https://doi.org/10.1080/03014223.2000.9517624
Tancell C, Sutherland WJ, Phillips RA (2016) Marine spatial planning for the conservation of albatrosses and large petrels breeding at South Georgia. Biol Cons 198:165–176. https://doi.org/10.1016/j.biocon.2016.03.020
Vavrek MJ, Vavrek MMJ (2020) Package fossil.
Wakefield ED, Phillips RA, Matthiopoulos J et al (2009) Wind field and sex constrain the flight speeds of central-place foraging albatrosses. Ecol Monogr 79:663–679. https://doi.org/10.1890/07-2111.1
Wakefield ED, Phillips RA, Trathan PN et al (2011) Habitat preference, accessibility and competition limiti the global distribution of breeding black-browed albatross. Ecol Monogr 81:141–167. https://doi.org/10.1890/09-0763.1
Wakefield ED, Phillips RA, Belchier M (2012) Foraging black-browed albatrosses target waters overlaying moraine banks - a consequence of upward benthic-pelagic coupling? Antarct Sci 24:269–280. https://doi.org/10.1017/S0954102012000132
Ward P, Zahavi A (1973) The importance of certain assemblages of birds as “information-centres” for food-finding. Ibis 115:517–534. https://doi.org/10.1111/j.1474-919X.1973.tb01990.x
Weimerskirch H, Mougey T, Hindermeyer X (1997) Foraging and provisioning strategies of black-browed albatrosses in relation to the requirements of the chick: natural variation and experimental study. Behav Ecol 8:635–643. https://doi.org/10.1093/beheco/8.6.635
Weimerskirch H, Guionnet T, Martin J et al (2000) Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proceedings of the Royal Society of London B: Biological Sciences 267:1869–1874. https://doi.org/10.1098/rspb.2000.1223
Weimerskirch H, Bertrand S, Silva J, et al (2010) Use of social information in seabirds: compass rafts indicate the heading of food patches. PLoS ONE 5:e9928. https://doi.org/10.1371/journal.pone.0009928
Wickham H, Muller K (2018) dplyr: A Grammar of Data Manipulation. R package version 0.7.6. https://CRAN.R-project.org/package=dplyr
Wilson L, McSorley CA, Gray CM et al (2008) Rafting behaviour of Manx shearwaters Puffinus puffinus. Seabird 21:85–93
Wilson LJ, McSorley CA, Gray CM et al (2009) Radio-telemetry as a tool to define protected areas for seabirds in the marine environment. Biol Cons 142:1808–1817. https://doi.org/10.1016/j.biocon.2009.03.019
Yoda K, Shiomi K, Sato K (2014) Foraging spots of streaked shearwaters in relation to ocean surface currents as identified using their drift movements. Prog Oceanogr 122:54–64. https://doi.org/10.1016/j.pocean.2013.12.002
Acknowledgements
We are grateful to all the field workers involved in the device deployment and retrieval and Andy Wood for database support. This study represents a contribution to the Ecosystems Component of the British Antarctic Survey Polar Science for Planet Earth Programme, funded by the Natural Environment Research Council (NERC).
Author information
Authors and Affiliations
Contributions
EKO and CKF should be considered joint first author. RAP conceived the project. CKF processed data prior to analysis. EKO conducted data analysis and EKO and CKF prepared the first manuscript draft with support from RAP. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kowalska O’Neil, E.W.M., Frankish, C.K. & Phillips, R.A. Rafting behaviour of albatrosses and petrels at South Georgia. Polar Biol 46, 597–610 (2023). https://doi.org/10.1007/s00300-023-03146-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03146-4