Abstract
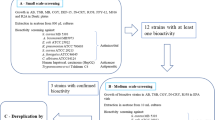
Sponges (Porifera) currently represent one of the richest sources of natural products and account for almost half of the pharmacologically active compounds of marine origin. However, to date very little is known about the pharmacological potential of the sponges from polar regions. In this work we report on screening of ethanolic extracts from 24 Antarctic marine sponges for different biological activities. The extracts were tested for cytotoxic effects against normal and transformed cell lines, red blood cells, and algae, for modulation of the activities of selected physiologically important enzymes (acetylcholinesterase, butyrylcholinesterase, and α-amylase), and for inhibition of growth of pathogenic and ecologically relevant bacteria and fungi. An extract from Tedania (Tedaniopsis) oxeata was selectively cytotoxic against the cancer cell lines and showed growth inhibition of all of the tested ecologically relevant and potentially pathogenic fungal isolates. The sponge extracts from Isodictya erinacea and Kirkpatrickia variolosa inhibited the activities of the cholinesterase enzymes, while the sponge extracts from Isodictya lankesteri and Inflatella belli reduced the activity of α-amylase. Several sponge extracts inhibited the growth of multiresistant pathogenic bacterial isolates of different origins, including extended-spectrum beta-lactamase and carbapenem-resistant strains, while sponge extracts from K. variolosa and Myxilla (Myxilla) mollis were active against a human methicillin-resistant Staphylococcus aureus strain. We conclude that Antarctic marine sponges represent a valuable source of biologically active compounds with pharmacological potential.

Similar content being viewed by others
References
Abbas S, Kelly M, Bowling J, Sims J, Waters A, Hamann M (2011) Advancement into the Arctic region for bioactive sponge secondary metabolites. Mar Drugs 9:2423–2437
Abdo DA, Motti CA, Battershill CN, Harvey ES (2007) Temperature and spatiotemporal variability of salicylihalamide A in the sponge Haliclona sp. J Chem Ecol 33:1635–1645
Ali H, Houghton PJ, Soumyanath A (2006) α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes, with particular reference to Phyllanthus amarus. J Ethnopharmacol 107:449–455
Avila C, Taboada S, Núňez-Pons L (2008) Antarctic marine chemical ecology: what is next? Mar Ecol 29:1–71
Bernfeld P (1955) Amylases, α and β. In: Colowick S, Kaplan N (eds) Methods in enzymology. Academic Press, Waltham, pp 149–158
Brezovšek P, Eleršek T, Filipič M (2014) Toxicities of four anti-neoplastic drugs and their binary mixtures tested on the green alga Pseudokirchneriella subcapitata and the cyanobacterium Synechococcus leopoliensis. Water Res 52:168–177
de Hoog GS, Guarro J, Gene J, Figueras MJ (2009) Atlas of clinical fungi. http://www.cbs.knaw.nl/index.php/atlas-of-clinical-fungi. Accessed 21 Oct 2015
Duckworth AR, Battershill CN (2001) Population dynamics and chemical ecology of New Zealand Demospongiae Latrunculia sp. nov. and Polymastia croceus (Poecilosclerida: Latrunculiidae: Polymastiidae). New Zeal J Mar Freshw Res 35:935–949
Ellman GL, Courtney D, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ferretti C, Vacca S, De Ciucis C et al (2009) Growth dynamics and bioactivity variation of the Mediterranean demosponges Agelas oroides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Mar Ecol 30:327–336
Fusetani N, Shinoda K, Matsunaga S (1993) Bioactive marine metabolites. 48. Cinachyrolide A: a potent cytotoxic macrolide possessing two spiro ketals from marine sponge Cinachyra sp. J Am Chem Soc 115:3977–3981
Garnica M, Nucci M (2013) Epidemiology of fusariosis. Curr Fungal Infect Rep 7:301–305
Gerwick WH, Moore BS (2012) Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol 19:85–98
Gopi M, Kumaran S, Kumar TT, Deivasigamani B, Alagappan K, Prasad SG (2012a) Antibacterial potential of sponge endosymbiont marine Enterobacter sp at Kavaratti Island, Lakshadweep Archipelago. Asian Pac J Trop Med 5:142–146
Gopi M, Ajith Kumar TT, Balagurunathan R, Vinoth R, Dhaneesh KV, Rajasekaran R, Balasubramanian T (2012b) Phylogenetic study of sponge-associated bacteria from the Lakshadweep Archipelago and the antimicrobial activities of their secondary metabolites. World J Microbiol Biotechnol 28:761–766
Gostinčar C, Turk M, Plemenitaš A, Gunde-Cimerman N (2009) The expressions of Δ9-, Δ12-desaturases and an elongase by the extremely halotolerant Hortaea werneckii are salt dependent. FEMS Yeast Res 9:247–256
Hood KA, West LM, Northcote PT, Berridge MV, Miller JH (2001) Induction of apoptosis by the marine sponge (Mycale) metabolites, mycalamide A and pateamine. Apoptosis 6:207–219
Hu GP, Yuan J, Sun L, She ZG, Wu JH, Lan XJ, Zhu X, Lin YC, Chen SP (2011) Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar Drugs 9:514–525
Janussen D, Downey RV (2014) Porifera. In: De Broyer C, Koubbi P, Griffiths HJ et al (eds) Biogeographic atlas of the Southern ocean. Scientific Committee on Antarctic Research, Cambridge, pp 94–102
Kaur J, Zhang MQ (2000) Molecular modelling and QSAR of reversible acetylcholinesterase inhibitors. Curr Med Chem 7:273–294
Kersken D, Göcke C, Brandt A, Lejzerowicz F, Schwabe E, Seefeldt AM, Veit-Köhler G, Janussen D (2014) The Infauna of three widely distributed sponge species (Hexactinellida and Demospongiae) from the deep Ekström Shelf in the Weddell-Sea, Antarctica. Deep Sea Res II 108:101–112
Laport MS, Santos OC, Muricy G (2009) Marine sponges: potential sources of new antimicrobial drugs. Curr Pharm Biotechnol 10:86–105
Leal MC, Puga J, Serôdio J, Gomes NCM, Calado R (2012) Trends in the discovery of new marine natural products from invertebrates over the last two decades—where and what are we bioprospecting? PLoS One 7:e30580
Lebar MD, Heimbegner JL, Baker BJ (2007) Cold-water marine natural products. Nat Prod Rep 24:774–797
Li B, Stribley JA, Ticu A, Xie W, Schopfer LM, Hammond P, Brimijoin S, Hinrichs SH, Lockridge O (2000) Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knock-out mouse. J Neurochem 75:1320–1331
Lippert H, Brinkmeyer R, Mülhaupt T, Iken K (2003) Antimicrobial activity in sub-Arctic marine invertebrates. Polar Biol 26:591–600
McClintock JB, Gauthier JJ (1992) Antimicrobial activities of Antarctic sponges. Antarctic Sci 4:179–183
McClintock JB, Amsler CD, Baker BJ, Van Soest RWM (2005) Ecology of Antarctic marine sponges: an overview. Integr Comp Biol 45:359–368
Miceli HM, Diaz AJ, Lee AS (2011) Emerging opportunistic yeast infections. Lancet Infect Dis 11:142–151
Molinski TF, Dalisay DS, Lievens SL, Saludes JP (2009) Drug development from marine natural products. Nat Rev Drug Discov 9:69–85
Munoz-Torrero D (2008) Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr Med Chem 15:2433–2455
Munro M, Blunt J, Dumdei E, Hickford S, Lill R, Li S, Battershill CN, Duckworth AR (1999) The discovery and development of marine compounds with pharmaceutical potential. J Biotechnol 70:15–25
Muricy G, Hajdu E, Araujo F V., Hagler AN (1993) Antimicrobial activity of Southwestern Atlantic shallow-water marine sponges (Porifera). In: Uriz MJ, Rützler K (eds) Recent advances in ecology and systematics of sponges, Scientia Marina. pp 427–432
Neofytos D, Horn D, De Simone JAJ (2007) Rhodotorula mucilaginosa catheter-related fungemia in a patient with sickle cell disease: case presentation and literature review. South Med J 100:198–200
Neumann K (ed) (2008) Marine-derived fungi: a source for structurally new and bioactive secondary metabolites. Dissertation, University of Bonn, Bonn
Novak Babič M, Zalar P, Ženko B et al (2015) Candida and Fusarium species known as opportunistic human pathogens from customer-accessible parts of residential washing machines. Fungal Biol 119:95–113
O’Donnell K, Sutton AD, Rinaldi GM et al (2010) Internet-accessible DNA sequence database for identifying Fusaria from human and animal infections. J Clin Microbiol 48:3708–3718
OECD (2011) Test no. 201: freshwater alga and cyanobacteria, growth inhibition test, OECD Guidelines for the testing of chemicals, section 2. OECD Publishing, Paris
Orhan IE (2013) Nature: a substantial source of auspicious substances with acetylcholinesterase inhibitory action. Curr Neuropharmacol 11:379–387
Page M, West L, Northcote P et al (2005) Spatial and temporal variability of cytotoxic metabolites in populations of the New Zealand sponge Mycale hentscheli. J Chem Ecol 31:1161–1174
Papaleo MC, Romoli R, Bartolucci G et al (2013) Bioactive volatile organic compounds from Antarctic (sponges) bacteria. New Biotechnol 30:824–838
Perry NB, Ettouati K, Litaudon M, Blunt JW, Munro MHG, Parkin S, Hope H (1994) Alkaloids from the Antarctic sponge Kirkpatrickia varialosa. Part 1: variolin B, a new antitumour and antiviral compound. Tetrahedron 50:3987–3992
Pezzementi L, Chatonnet A (2010) Evolution of cholinesterases in the animal kingdom. Chem Biol Interact 187:27–33
Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Meis JF, Gould IM, Fu W, Colombo AL, Rodriguez-Noriega E (2007) Results from the ARTEMIS DISK global antifungal surveillance study, 1997–2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol 45:1735–1745
Pohanka M (2011) Cholinesterases, a target of pharmacology and toxicology. Biomed Pap 155:219–223
Sacristán-Soriano O, Banaigs B, Becerro MA (2012) Temporal trends in the secondary metabolite production of the sponge Aplysina aerophoba. Mar Drugs 10:677–693
Sagar S, Kaur M, Radovanovic A, Bajic VB (2013) Dragon exploration system on marine s ponge compounds interactions. J Cheminform 5:11
Sales PM, Souza PM, Simeoni LA, Silveira D (2012) α-Amylase inhibitors: a review of raw material and isolated compounds from plant sources. J Pharm Pharm Sci 15:141–183
Scannapieco FA, Torres G, Levine MJ (1993) Salivary α-amylase: role in dental plaque and caries formation. Crit Rev Oral Biol Med 4:301–307
Schlitzer R (2015) Ocean data view. http://odv.awi.de. Accessed 2 Nov 2015
Schmitz FJ, Gunasekera SP, Yalamanchili G, Hossain MB, Van der Helm D (1984) Tedanolide: a potent cytotoxic macrolide from the Caribbean sponge Tedania ignis. J Am Chem Soc 106:7251–7252
Sepčić K, Kauferstein S, Mebs D, Turk T (2010) Biological activities of aqueous and organic extracts from tropical marine sponges. Mar Drugs 8:1550–1566
Shaaban M, Abd-Alla HI, Hassan AZ, Aly HF, Ghani MA (2012) Chemical characterization, antioxidant and inhibitory effects of some marine sponges against carbohydrate metabolizing enzymes. Org Med Chem Lett 16:30
Simone M, Erba E, Damia G, Vikhanskaya F et al (2005) Variolin B and its derivate deoxy-variolin B: new marine natural compounds with cyclin-dependent kinase inhibitor activity. Eur J Cancer 41:2366–2377
Singh AJ, Xu CX, Xu X, West LM, Wilmes A, Chan A, Hamel E, Miller JH, Northcote PT, Ghosh AK (2010) Peloruside B, a potent antitumor macrolide from the New Zealand marine sponge Mycale hentscheli: isolation, structure, total synthesis, and bioactivity. J Org Chem 75:2–10
Sutton DA, Brandt ME (2011) Fusarium and other opportunistic hyaline fungi. In: Versalovic J, Jorgensen JH, Funke G, et al. (eds) Manual of clinical microbiology, 10th Edition. American Society of Microbiology, pp 1853–1879
Taboada S, García-Fernández LF, Bueno S, Vázquez J, Cuevas C (2010) Antitumoral activity in Antarctic and sub-Antarctic benthic organisms. Antarctic Sci 22:494–507
Thakur NL, Anil AC (2000) Antibacterial activity of the sponge Ircinia ramosa: importance of its surface-associated bacteria. J Chem Ecol 26:57–71
Thompson JE, Murphy PT, Berquist PR, Evans EA (1987) Environmentally induced variation in diterpene composition of the marine sponge Rhopaloeides odorabile. Biochem Syst Ecol 15:595–606
Timm C, Mordhorst T, Kock M (2010) Synthesis of 3-alkyl pyridinium alkaloids from the arctic sponge Haliclona viscosa. Mar Drugs 8:483–497
Turk T, Ambrožič Avguštin J, Batista U et al (2013) Biological activities of ethanolic extracts from deep-sea antarctic marine sponges. Mar Drugs 11:1126–1139
van Asbeck EC, Clemons KV, Stevens DA (2009) Candida parapsilosis: a review of its epidemiology, clinical aspects, typing and antimicrobial susceptibility. Crit Rev Microbiol 35:283–309
Xin Y, Kanagasabhapathy M, Janussen D, Xue S, Zhang W (2011) Phylogenetic diversity of Gram-positive bacteria cultured from Antarctic deep-sea sponges. Polar Biol 34:1501–1512
Zalar P, Novak M, de Hoog GS, Gunde-Cimerman N (2011) Dishwashers—a man-made ecological niche accommodating human opportunistic fungal pathogens. Fungal Biol 115:997–1007
Zhen Z, Jing Z, Caihuan KE, Dexiang W (2013) Antimicrobial activities of novel cultivable bacteria isolated from marine sponge Tedania anhelans. Chin J Oceanol Limnol 31:581–590
Acknowledgments
The authors gratefully acknowledge the Slovenian Research Agency (Research Programmes P1-0207, P4-0127, P1-0055, and P1-0198), the ERASMUS Student Mobility Programme for financial support to MK, LS and ML, and Deutsche Forschungsgemeinschaft for financial support for the Antarctic sponge research project by DJ (JA-1063/17-1). We acknowledge the financial support received from the Ministry of Education, Science and Sport and the University of Ljubljana via the “Innovative scheme for co-financing of doctoral studies,” the Slovenian Research Agency through the Infrastructural Centre Mycosmo, MRIC UL, and the Centre of Excellence for Integrated Approaches in Chemistry and Biology of Proteins (CIPKeBiP). Dr. Chris Berrie is greatly acknowledged for editing and appraisal of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Berne, S., Kalauz, M., Lapat, M. et al. Screening of the Antarctic marine sponges (Porifera) as a source of bioactive compounds. Polar Biol 39, 947–959 (2016). https://doi.org/10.1007/s00300-015-1835-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1835-4