Abstract
The consequences of global warming are particularly evident in high polar areas. Deglaciation phenomenon—negative mass balance of Svalbard glaciers and recession of tidal glaciers—results in landscape and shoreline change. These areas of very dynamic conditions are now open for primary colonists, among them hydroids, typical early colonists of the vacant substratum. This study aims to explore the patterns of Hydrozoan diversity and distribution in Hornsund (west Spitsbergen). Hydroids associated with shallow water kelp beds as well as those occurring on deeper subtidal soft bottom were collected at sites located along gradients of glacial disturbance (i.e., high mineral sedimentation, ice-berg scouring). Samples were collected by scuba diving (three sites of different distance to active tidal glaciers), van Veen grabs (two sites located in the inner and outer fjord basin), and dredges taken from along a fjord transect. Hydroid diversity differed significantly between sites located in the vicinity of glaciers fronts in glaciated bays and sites comparatively free from glacier disturbance. Glacial disturbance results in low frequencies of occurrence and high levels of rarity of hydroids at sites located close to glacier fronts. The species richness of hydroids colonizing the hard substrate elements present in deeper subtidal decreases along the fjord axis (i.e., along the glacial sedimentation gradient).
Similar content being viewed by others
Introduction
Arctic fjord ecosystems are strongly affected by glacier-derived disturbances mainly derived from the outflow of meltwaters produced by active tidewater glaciers. Factors that affect marine biota include large fresh-water inputs, high levels of concentrations of mineral suspensions in waters, high rates of inorganic sedimentation, and iceberg bottom-scouring (Węsławski et al. 1995; Hop et al. 2002; Włodarska-Kowalczuk and Pearson 2004).
Increased tidewater glacier activity is predicted to be one of the consequences of global warming (Lefauconnier et al. 1994; Svendsen et al. 1996; Włodarska-Kowalczuk and Węsławski 2001). High loads of inorganic material increase water turbidity which influences light conditions and reduces primary production. Furthermore, high levels of inorganic particle sedimentation in the vicinity of glacier outlets “dilute” the organic matter available to benthic consumers (Görlich et al. 1987). The presence of sediment in the water column is regarded as a severe stress agent for hard-bottom macroorganisms, especially suspension feeders (Moore 1977; Görlich et al. 1987; Kukliński 2002; Airoldi 2003). Suspension-feeding organisms in sediment-stressed environments are observed to experience reduced survival and mortality as a consequence of burial, scouring, and clogging of their filtering apparatus by sediment (Moore 1977). This can cause changes in species composition and diversity in communities. Moreover, the distribution patterns of sessile invertebrates are formed by substrate selection processes occurring during larval settlement (Wilson 1968; Hayward 1980). In a sediment-impacted environment, the loss of suitable hard substrates can inhibit larval recruitment (Genzano et al. 2002; Irving and Connel 2002; Airoldi 2003).
To study the effect of glacier-derived impact in a glaciated fjord, we chose benthic hydroids as a model group. Hydroids are a poorly known group in Svalbard waters and species new to science and to the region are still being described (Ronowicz 2007; Ronowicz and Schuchert 2007; Voronkov et al. 2010). Despite their small size and delicate forms, hydrozoans play a significant role in the environment (e.g., bioconstructing, energy transfer) and can be useful for monitoring purposes. Their sessile mode of life and phenotypic variations within species make them good indicators of their environmental surroundings (Gili and Hughes 1995). It has been recognized that hydroids are an important component of epibiontic communities in Arctic fjords (Włodarska-Kowalczuk et al. 2009). Hydrozoa represent the third most species-rich group (after Polychaeta and Bryozoa) and together with bryozoans constitute about half of the species present within the kelp forest assemblage in a Svalbard fjord (Włodarska-Kowalczuk et al. 2009). Hydroids are typical early colonists of vacant substrates (Hughes et al. 1991) and play an important role in pelagic-benthic coupling (Gili et al. 1998). They transfer energy from the pelagic to benthic systems (Gili et al. 1998; Bouillon et al. 2004) and viceversa (Orejas et al. 2000). It is documented that presence of hydroids increases the complexity of habitats and enriches benthic communities in relation to the diversity and abundance of benthic fauna (Bradshaw et al. 2003). Hydroid erect colonies can serve as a shelter from predators, nursery grounds, as a substrate for egg deposition or as a physical support for suspension feeders (e.g., encrusting bryozoans, juvenile bivalves) which are lifted up above the sea bottom where current conditions are more favorable and where they are less exposed to sedimentation.
The present study explores the effects of glacial disturbance on the patterns of hydrozoan distribution and diversity in two habitats of an Arctic glacial fjord, the kelp beds, and deeper subtidal region.
Study area
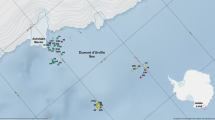
The study was performed in a high Arctic fjord—Hornsund (76°56′–77°03′N; 15°28′–16°45′E), the southernmost fjord in Spitsbergen (Svalbard Archipelago) (Fig. 1).
The area of the fjord is 1,232 km2. Its wide entrance (12 km) is oriented toward the west and its broad connection with the open shelf and the lack of a sill enable the penetration of oceanic waters into the central basin of the fjord. The hydrological regime in Hornsund is complex and is formed by the interplay of warm waters transported by the West Spitsbergen Current, cold waters transported by the Sørkapp Current, and coastal waters that develop locally and seasonally (Swerpel 1985).
Hornsund is a typical glacial fjord, where ice cliffs constitute 24% of the coastline. Thirteen tidewater glaciers terminate in Hornsund waters (Głowacki 2007). These glaciers strongly modify the physical environment of the fjord by discharging high loads of fresh water and inorganic suspensions. The fjord expanded continually due to the recession of all tidal glaciers. One of the best studied glaciers, Hornbreen, retreated by an average of 140 m annually from 1900 to 1990 (Ziaja 2001). Hansbreen, a glacier located in the vicinity of the Polish Polar Station, has gradually lost 0.5 m of its thickness annually for the last few years because of calving from ice front and evaporation (Głowacki 2007). Active tidewater glaciers discharge icebergs in summer. The commonly used in this article term “glaciated bay” refers to a bay that is located in a vicinity of a tidal glacier and remains under the strong influence of tidal glacier activity.
Methods
Sampling
Hydroids of kelp beds
Hydrozoans inhabiting kelp beds were collected by SCUBA divers at three sites located at different distances to active glacier outflows in July 2003 in Hornsund fjord (Table 1; Fig. 2):
-
Isbjørnhamna (I)—situated close to the fjord entrance but close to the Hansbreen glacier outflow, with very high inorganic particle sedimentation;
-
Hyrneodden (H)—situated in the inner part of the fjord, close to several active tidewater glacier outflows, with an intermediate level of inorganic particle sedimentation;
-
Gåshamna (G)—situated close to the fjord entrance, far from the glacier outflows, with very low inorganic particle sedimentation.
Altogether 340 samples of the three most common macroalgae Laminaria digitata (Hudson) Lamouroux, 1813, Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders, 2006, and Alaria esculenta (Linnaeus) Greville, 1830 with associated fauna were collected: 133 samples in Isbjørnhamna, 113 samples in Gåshamna, and 94 samples in Hyrneodden. Samples were collected at each site from depths of 5 and 10 m. Each algal sample was carefully cut from the substrate with a knife and placed in a separate bag of 1 mm mesh size.
Sedimentation rates at all three sites were determined using sediment traps made of PVC cylinders (height—50 cm, diameter—8 cm) exposed for 24 h (3 replicates at each site). After 24 h, they were recovered from the sea bed and taken to the laboratory and left for another 24 h until the sediments had settled. Then, the supernatant was decanted, and the remaining mixture of water and sediment was filtered through Whatman GF/C 47 mm filters. These were dried at 60°C for 24 h and weighed. The weighed filters loaded with sediment were placed in an oven (450°C) for 24 h and afterward weighed again to determine the mineral matter weight and organic matter loss.
Hydroids of deeper subtidal
The material collected in deeper subtidal zones included samples taken with van Veen grabs and dredges (Table 1; Fig. 2). Samples were collected from aboard the r/v Oceania. Grab sampling was performed in Hornsund in July 2005 at two sites: the fjord inner basin, Brepollen, and at the outer part on the southern bank of the fjord entrance (Fig. 2). Six stations were arranged at 200-m intervals along 1-km transects at both locations. Four replicates were collected at each station using a van Veen grab (0.1 m2). Station depths varied from 100 to 120 m on the inner transect and from 100 to 115 m on the outer transect.
Six samples were collected by dredging along the fjord axis in July 2002 (Fig. 2).
All samples were sieved gently through a sieve of 0.5 mm mesh size and fixed in a 4% formaldehyde solution aboard the ship. Material was sorted and all the hydroid specimens were identified in the laboratory to the lowest possible taxonomic level.
Data analyses
To assess and compare, the species richness of the three kelp beds sites species accumulation curves with 95% confidence intervals were computed using formulae by Colwell et al. (2004). The computation of confidence intervals allows for the direct statistical comparison of the species richness of two data sets, i.e., differences are not significant at P < 0.05 if the 95% confidence intervals overlap (Colwell et al. 2004).
The number of species per sample was used as the measure of sample species richness. The differences between sample species richness among sites (for diving sites and grab samples estimated separately) were tested using the nonparametric Kruskal–Wallis test. The pair-wise Mann–Whitney U-test was used for post-hoc multiple comparisons (STATISTICA v. 6, Statsoft).
The number of rare species (uniques—occurring in one sample and duplicates—occurring in two samples) for each site was calculated, and the percentage of rare species in the total number of species was assessed for each site. The frequency of species occurrence at sites was estimated as the percentage of samples at particular sites in which the species occurred to the number of samples at the sites. Then the mean frequency of hydroid occurrence was determined for each site as the mean value of frequencies calculated for all species occurring at a site. Multivariate analysis was performed to identify patterns of hydroid species distribution. The similarity among samples was calculated with the Bray-Curtis index. The differences in species composition among the three sites were tested with the one-way ANOSIM pair-wise test performed with the Primer package v. 6 (Clarke and Warwick 2001).
The frequency of species occurrences in samples collected with the use of diving and grabs was estimated as the percentage of samples in which the species occurred to the total number of samples at the site.
Results
Hydroids of kelp beds
Three sites were located at different distances to active tidal glaciers and differed with respect to glacier disturbance levels. There were clear differences in sedimentation rates of mineral particles. Both mineral and organic sedimentation rates were highest in Isbjørnhamna—the most affected by the tidal glacier (146.53 mg dm−3 day−1 ± SD 103.68 mg dm−3 day−1 and 20.36 mg dm−3 day−1 ± SD 12.24 mg dm−3 day−1, respectively) and reached the lowest value in Gåshamna—the site free from direct impact from glaciers (12.73 mg dm−3 day−1 ± SD 4.75 mg dm−3 day−1, and 5.27 mg dm−3 day−1 ± SD 2.59 mg dm−3 day−1, respectively). Numerous icebergs (including large grounded ones) were observed at Hyrneodden during the entire sampling period (5 weeks), while at Isbjørnhamna, the icebergs were observed in lower numbers and only occasionally. No icebergs were observed at Gåshamna.
Hydroids were present on collected macroalgal thalli occurring with a frequency of 79% in samples at Gåshamna, 63% at Isbjørnhamna, and 28% at Hyrneodden.
The species accumulation curves plotted against the sampling effort for the three sites did not stabilize toward asymptotic values (Fig. 3). The 95% confidence intervals for the observed number of taxa at Isbjørnhamna and Gåshamna overlap indicating no significant difference in the total species richness between the two sites. The total number of species was the lowest in Hyrneodden and differed significantly from the other two sites.
The maximum total species richness was noted at Isbjørnhamna (23 species) (Fig. 4). However, there were 12 species occurring at low numbers (uniques and duplicates). A total of 19 species were observed at Gåshamna (with three species at low number of records), while only ten species were recorded at Hyrneodden (with half of them being uniques or duplicates—see “Methods”) (Table 2).
There were significant differences in hydroid sample species richness among the studied sites (Kruskal–Wallis test: H = 83.85, P < 0.05). The post-hoc pair-wise tests revealed significant differences in sample species numbers between two pairs of sites: Gåshamna—Isbjørnhamna and Gåshamna—Hyrneodden (Mann–Whitney U-test, P < 0.05), whereas hydroid sample species richness at Isbjørnhamna and Hyrneodden did not differ significantly (Mann–Whitney U-test: P > 0.05).
The highest mean sample species richness was found at Gåshamna (Fig. 5).
The highest mean frequency of hydroid species occurrences was also noted at Gåshamna (10.52%), while at Isbjørnhamna and Hyrneodden values were lower (3.79 and 2.55%, respectively). The most common species occurred with the frequencies exceeding 20% at Gåshamna and Isbjørnhamna, while no species were recorded with frequency over 10% at Hyrneodden (Table 3).
Hydroids in deeper subtidal
Hydroids were present in 79% of grab samples taken at the Outer stations and in 7% of samples taken at the Inner stations. The total number of observed hydroids taxa was 20. All of them were recorded at the Outer site (fjord entrance), while at the Inner site (glaciated bay) only two species were noted (Fig. 4). Most of the species (18 species) belonged to the order Leptothecata. Anthoathecata was represented by only two species (E. annulatum and Sarsia sp.) present only at the Outer site.
Sample species richness varied significantly between the two sites (Kruskal–Wallis test: H = 23.58, P < 0.05) and reached on average 5.5 species per sample at the Outer transect and 0.1 species per sample at the Inner transect (Fig. 6).
Hydroid species composition varied between samples of the Inner and Outer sites. Symplectoscyphus tricuspidatus was the most common species at the Outer site (frequency 67%) (Table 3). Abietinaria pulchra, Eudendrium annulatum, Sertularia schmidti, and Sertularia cupressoides) occurred at frequencies over 50%. At the Inner site, both Gonothyraea loveni and Lafoeina maxima occurred with 7% frequency.
Most of the specimens collected were not attached to any substrate. Due to the sampling methods delicate colonies of hydroids are often broken off, although if the coenosarc and hydrant are present the specimen was treated as live-collected. Among the few that were found with substrata, they attached to rocks, bryozoans, two species of other hydrozoans, shells of bivalves and barnacles.
In dredge samples, species richness changed according to the location within the fjord transect. It was lowest (one and zero species per sample) for stations (F and G respectively) in the glaciated bay and sharply rising with increasing distance from the inner fjord. The highest values were at stations A and B (14 and 15 species per sample correspondingly) at the fjord entrance (Fig. 4). The only species collected in the inner fjord was S. argentea. Only one representative of the order Anthoathecata, E. annulatum, occurred exclusively at the outer stations.
The differences in species composition among the sites under the influence of glacial impact (Inner, F and G) and those located in the outer part of the fjord, not affected by the glacial impact (Outer, A, B, D, and E), were significant and high (one-way ANOSIM pair-wise test: R = 0.58, P = 0.01).
Discussion
Arctic fjord ecosystems are strongly affected by glacier-derived disturbances, mainly by the outflow of meltwater produced by active tidewater glaciers. Tidewater glaciers are often situated in the innermost parts of the fjords (in Hornsund the majority of glaciers), and they have a strong effect on the physical regimes of whole basins and can impact hard and soft-bottom benthic communities (Farrow et al. 1983; Syvitski et al. 1989; Węsławski et al. 1995; Kukliński 2002; Włodarska-Kowalczuk and Pearson 2004). Species richness decreases along the fjord from the outer part to the inner, indicating that hydroids are vulnerable to environmental stress glacier-derived.
Hydroids in kelp beds
The three sites studied (Isbjørnhamna, Hyrneodden, Gåshamna) differ with regard to hydroid species richness; however, the patterns are different for richness assessed on the scale of single samples (alpha diversity) and on that of whole sites (gamma diversity). The comparison of hydroid sample species richness reveals significant differences between the sites influenced by the glaciers (Isbjørnhamna and Hyrneodden) and the Gåshamna site that is free of glacial impact.
Sample species richness is lower at the two sites situated in the vicinity of glaciers (Isbjørnhamna and Hyrneodden) than at the site located far from glacial inflows (Gåshamna). The impact of sedimentation is the most likely reason for the differences in alpha species richness among the sites studied. A similar decrease in macrobenthic species richness and diversity caused by sedimentation has been observed in soft bottom (Włodarska-Kowalczuk et al. 2005) and rocky bottom (Airoldi 2003) communities. The distribution of hydroid species was significantly related to the amount of silt on the surface of substrate (Voronkov et al. 2010). Magurran (2004) reports that sample species richness is a very sensitive indicator of community perturbation and recommends using sample species richness measures in environmental assessment studies.
Surprisingly, the total number of species per site (gamma diversity) is only lower at Hyrneodden in the inner fjord adjacent to glaciers. There are no significant differences between the other two sites (Isbjørnhamna and Gåshamna) that have different sedimentary regimes, but they are similar in that they are located in the outer part of the fjord. Differences in gamma diversity at different locations in the fjord can be explained by the distance to the fjord entrances and to the local hydroid species pool. According to Buhl-Mortensen and Høisæter (1993), the species pools of fjordic communities are formed by “…the faunal assemblage outside the fjords, from which the hypothetical fjord colonizers might be recruited.” Since there is no sill at the entrance to the fjord, larvae can enter Hornsund. The outer part of the fjord is more accessible to new hydroid recruits. The distance from Hyrneodden (inner fjord site) to the species pool outside the fjord might be too far for some larvae to cover since hydroid planula larvae typically remain in the water column only a few hours to a few days (Cornelius 1992). Additionally, the gradient of physical conditions from offshore to the inner fjords (oceanic waters modified by glacial melt waters) may represent a “habitat barrier” for the larvae. Klitgaard-Kristensen and Buhl-Mortensen (1999) report that decreases in amphipod and mollusk diversity observed in Norwegian fjords along fjord transects is a result of progressively less favorable environmental conditions towards the fjord head. Environmental conditions such as sediment type, bottom currents, ice scouring, and the quantities and availability of organic carbon change along fjord axes in a way that allows fewer species to thrive in inner basins.
Differing patterns of hydroid frequencies are observed at the three study sites. Half of the species occurring at Isbjørnhamna (52%) and Hyrneodden (50%) are recorded only once or twice, while at Gåshamna rare species comprise only 16% of the species present. The mean hydroid species frequency is higher at Gåshamna in comparison with other sites indicating that species occur more often in the samples collected at this unimpacted site. High sedimentation rates and iceberg scouring at sites situated close to the glacial front presumably restrict hydroid colonization which, in turn, resulted in the low frequencies of occurrence and high levels of rarity there. Thus, species rarity occurs because environmental factors constrain their ranges to small areas and do not permit them to establish wider distributions (Hill and Keddy 1992; Gaston 1994; Greulich et al. 2000). Reasons for rare occurrence may include the following: (1) lack of available habitats corresponding to species requirements; (2) lack of the chances for successful settlement that depend on larval dispersal capacity and establishment ability; (3) lack of the ability of developing into mature stages, and withstanding given physical conditions and biological interactions; (4) lack of reproductive success (Greulich et al. 2000). Since there is no direct glacier impact at Gåshamna, sedimentation rates are low and no iceberg scouring occurs. Thus, the hydroid community here is undisturbed and is older because of the low frequency of physical disturbances.
Hydroids of deeper subtidal
The total species richness and sample species richness of Hydrozoa collected with grabs and dredges in the deeper sublittoral zone decreases from the outer to the inner parts of the fjord. Studies of hydroids on hard bottom substrate from Kongsfjorden (Spitsbergen) seem to confirm the finding as reduced diversity and biomass in the inner part of the fjord compared to the outer part was observed (Voronkov et al. 2010). Only three species (S. argentea, G. loveni, L. maxima) are present within the innermost glaciated bay, where their occurrence is limited to single records. These three species are characterized by an erect colony morphology that allows them to rise above the sediments and avoid being buried. Their presence in such unfavorable conditions is most likely accidental. Suspended silt is probably a detrimental for hydroids (Round et al. 1961). Withers and Thorpe (1977) suggest that sediment blanketing surfaces might inhibit larval settling or metamorphosis in most species. Another factor limiting hydroid colonization in inner fjordic basins is the lack of hard substrates. The only hard substrates available in the inner parts of fjords are mollusk shells or drop stones, which are likely to be covered by sediment. Soft bottoms and glacial influence can limit hydroid colonization potential and persistence in specific habitats of glaciated bays.
The phenomenon of decreasing species richness and/or occurrence from the outer to the inner parts of fjords is observed throughout the macrobenthic community (Włodarska-Kowalczuk et al. 2005; Renaud et al. 2007) and for many different macrofaunal groups, including bryozoans (Kukliński et al. 2005), sipunculans (Kędra and Włodarska-Kowalczuk 2008), and crustaceans (Legeżyńska, personal communication). Nevertheless, glaciated bays are not deprived of life. The characteristic species for the soft bottom glacial community include bivalves from the families Nuculanidae and Thyasiridae, which are very efficient detritus feeders, and the polychaete Chone paucibranchiata (Krøyer 1856) which feeds both on organic particles suspended in waters and on sediment surfaces (Włodarska-Kowalczuk and Pearson 2004). High inorganic sedimentation is not a limiting factor for the suspension-feeding bryozoan Alcyonidium disciforme Smitt 1871, a common inhabitant of glaciated bays. The sand particles from its immediate surroundings are sequestered and bound in an external cuticle (Kukliński and Porter 2004) that is likely an adaptation, but one that is not yet fully understood.
Conclusions
Our results indicate that the species richness of hydroids decreases along the fjord axis (i.e., along the glacial sedimentation gradient). The lack of hard substrate together with a very high environmental stress are the major limiting forces for the occurrence of hydroids in the deeper sublittoral zone of the inner fjord. Severe disturbance (high sedimentation rates and frequent iceberg scouring) results in the low frequencies of occurrence and high levels of rarity of hydroids at sites located close to glacier fronts.
Changes in the marine environment in Arctic fjords (increased tidal glacier activity and increased load of mineral particles) induced by climate change may result in the decrease of hydroid diversity.
References
Airoldi L (2003) The effect of sedimentation on rocky coast assemblages. Oceanogr Mar Biol Annu Rev 41:161–236
Bouillon J, Medel MD, Pages F, Gili JM, Boero F, Gravili C (2004) Fauna of the Mediterranean Hydrozoa. Sci Mar 68:5–438
Bradshaw C, Collins P, Brand AR (2003) To what extent does upright sessile epifauna affect benthic biodiversity and community composition? Mar Biol 143:783–791
Buhl-Mortensen L, Høisæter T (1993) Mollusc fauna along an offshore-fjord gradient. Mar Ecol Progr Ser 97:209–224
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Cornelius PFS (1992) Medusa loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. Sci Mar 56:245–261
Farrow GE, Syvitski JPM, Tunnicliffe V (1983) Suspended particulate loading on the macrobenthos in a highly turbid fjord Knight Inlet, British Columbia Canadian. J Fish Aquat Sci 40:273–288
Gaston KJ (1994) Rarity. Chapman and Hall, London
Genzano NG, Zamponi MO, Excoffon AC, Acuňa FH (2002) Hydroid populations from sublittoral outcrops off Mar del Plata, Argentina: abundance, seasonality and reproductive periodicity. Ophelia 56:161–170
Gili JM, Hughes RG (1995) The ecology of marine benthic hydroids. Oceanogr Mar Biol Annu Rev 33:351–426
Gili JM, Alvà V, Coma C et al (1998) The impact of small benthic passive suspension feeders in shallow marine ecosystems: the hydroids as an example. Zool Verh Leiden 323:99–105
Głowacki P (2007) Role of physical and chemical processes in the internal structure formation and mass circulation of Spitsbergen glaciers. Publications of the Institute of Geophysics Polish Academy of Sciences, M-30(400) (in Polish)
Görlich K, Węsławski JM, Zajączkowski M (1987) Suspension settling effect on macrobenthos biomass distribution in the Hornsund fjord, Spitsbergen. Polar Res 5:175–192
Greulich S, Bornette G, Amoros C (2000) Persistence of a rare aquatic species along gradients of disturbance and sediment richness. J Veg Sci 11:415–424
Hayward PJ (1980) Invertebrate epiphytes of coastal marine algae. In: Price JH et al (eds) The shore environment, 2: ecosystems. Academic Press, London, New York, pp 761–787
Hill NN, Keddy PA (1992) Prediction of rarities from habitat variables: coastal plain plants on Nova Scotian lakeshores. Ecology 73:1852–1859
Hop H, Pearson T, Hegseth EN et al (2002) The marine ecosystem of Kongsfjorden, Svalbard. Polar Res 21:167–208
Hughes RG, Johnson S, Smith ID (1991) The growth patterns of some hydroids that are obligate epiphytes of seagrass leaves. Hydrobiologia 216(217):205–210
Irving AD, Connel SD (2002) Sedimentation and light penetration interact to maintain heterogeneity of subtidal habitats: algal versus invertebrate dominated assemblages. Mar Ecol Progr Ser 245:83–91
Kędra M, Włodarska-Kowalczuk M (2008) Distribution and diversity of sipunculan fauna in high Arctic fjords (west Svalbard). Polar Biol 31:1181–1190
Klitgaard-Kristensen D, Buhl-Mortensen L (1999) Benthic foraminifera along an offshore-fjord gradient: a comparison with amphipods and molluscs. J Nat Hist 33:317–350
Kukliński P (2002) Fauna of Bryozoa from Kongsfjorden, West Spitsbergen. Pol Polar Res 23:193–206
Kukliński P, Porter JS (2004) Alcyonidium disciforme: an exceptional Arctic bryozoan. J Mar Biol Assoc UK 84:267–275
Kukliński P, Gulliksen B, Lønne OJ, Węsławski JM (2005) Composition of bryozoan assemblages related to depth in Svalbard fjords and sounds. Polar Biol 28:619–630
Lefauconnier B, Hagen JO, Rudant JP (1994) Flow speed and calving rate of Kongsbreen glacier, 70°N Spitsbergen, Svalbard using SPOT images. Polar Res 13:59–66
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden
Moore PG (1977) Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanogr Mar Biol Annu Rev 15:225–363
Orejas C, Gili JM, Alvà V, Arntz W (2000) Predatory impact of an epiphytic hydrozoan in an upwelling area in the Bay of Coliumo (Dichato, Chile). J Sea Res 44:209–220
Renaud PE, Włodarska-Kowalczuk M, Trannum H, Holte B, Węsławski JM, Cochrane S, Dahle S, Gulliksen B (2007) Multidecadal stability of benthic community structure in a high-Arctic glacial fjord (van Mijenfjord, Spitsbergen). Polar Biol 30:295–305
Ronowicz M (2007) Benthic hydroids (Cnidaria: Hydrozoa) from Svalbard waters - biodiversity and distribution. J Mar Biol Assoc UK 87:1089–1094
Ronowicz M, Schuchert P (2007) Halecium arcticum, a new hydroid from Spitsbergen (Cnidaria: Hydrozoa). Zootaxa 1549:55–62
Round FE, Sloane JF, Ebling FJ, Kitching JA (1961) The ecology of Lough Ine. X. The hydroid Sertularella operculata (L.) and its associated flora and fauna: effects of transference to sheltered water. J Ecol 49:617–629
Svendsen JI, Elverhoi A, Mangerud J (1996) The retreat of the Barents Sea ice sheet on the western Svalbard margin. Boreas 25:244–256
Swerpel S (1985) The Hornsund Fiord: water masses. Pol Polar Res 6:475–496
Syvitski JPM, Farrow GE, Atkinson RJA, Moore PG, Andrews JT (1989) Baffin Island fjord macrobenthos: bottom communities and environmental significance. Arctic 42:232–247
Voronkov A, Stepanjants SD, Hop H (2010) Hydrozoan diversity on hard bottom in Kongsfjorden, Svalbard. J Mar Biol Assoc UK 90:1337–1352
Węsławski JM, Koszteyn J, Zajączkowski M, Wiktor J, Kwaśniewski S (1995) Fresh water in Svalbard fjord ecosystems. In: Skjodal HR et al (eds) Ecology of fjords and coastal waters. Elsevier Science BV, Amsterdam, pp 229–241
Wilson DP (1968) The settlement behaviour of the larvae of Sabellaria alveolata L. J Mar Biol Assoc UK 48:387–435
Withers RG, Thorpe H (1977) Studies on shallow sublittoral epibenthos of Langstone Harbour, Hampshire, using settlement panels. In: Keegan BF, Ceidigh PO (eds) Biology of benthic organisms. Pergamon, Oxford, pp 595–604
Włodarska-Kowalczuk M, Pearson T (2004) Soft-bottom macrobenthic faunal associations and factors affecting species distributions in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Polar Biol 27:155–167
Włodarska-Kowalczuk M, Węsławski JM (2001) Impact of climate warming on Arctic benthic biodiversity: a case study of two Arctic glacial bays. Clim Res 18:127–132
Włodarska-Kowalczuk M, Pearson TH, Kendall M (2005) Benthic response to chronic natural physical disturbance by glacial sedimentation in an Arctic Fjord. Mar Ecol Progr Ser 303:31–41
Włodarska-Kowalczuk M, Kukliński P, Ronowicz M, Legeżyńska J, Gromisz S (2009) Assessing species richness of macrofauna associated with macroalgae in Arctic kelp forests (Hornsund, Svalbard). Polar Biol 32:897–905
Ziaja W (2001) Glacial recession in Sorkappland and central Nordenskioldland, Spitsbergen, Svalbard, during the 20th century. Arct Antarct Alp Res 33:36–41
Acknowledgments
Special thanks to Dr. Peter Schuchert (Natural History Museum of Geneva) for taxonomic consultation of problematic material as well as to Suzanne Williams (Natural History Museum in London) for linguistic improvements. We acknowledge the constructive comments of anonymous referees that helped to improve the manuscript. The study has been undertaken thanks to the funds provided by grants from the Polish State Committee for Scientific Research nr 593/N-AODP/2009/0 and 522/N-NaGISA-GREN/2009/0.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ronowicz, M., Włodarska-Kowalczuk, M. & Kukliński, P. Patterns of hydroid (Cnidaria, Hydrozoa) species richness and distribution in an Arctic glaciated fjord. Polar Biol 34, 1437–1445 (2011). https://doi.org/10.1007/s00300-011-0999-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-0999-9