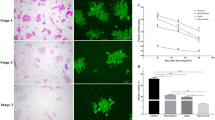
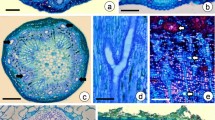
Abstract
Main conclusion
CgPG21 is mainly located in the cell wall, participates in the intercellular layer degradation of the cell wall during the formation of secretory cavity in the intercellular space-forming and lumen-expanding stages.
Abstract
The secretory cavity is a common structure in Citrus plants and is the main site for synthesis and accumulation of medicinal ingredients. The secretory cavity is formed in lysogenesis, when epithelial cells enter a process of programmed cell death. Pectinases are known to be involved in degradation of the cell wall during the cytolysis of secretory cavity cells, but the changes in cell structure, the dynamic characteristics of cell wall polysaccharides and the related genes regulating cell wall degradation are unclear. In this study, electron microscopy and cell wall polysaccharide-labeling techniques were used to study the main characteristics of cell wall degradation of the secreting cavity of Citrus grandis ‘Tomentosa’ fruits. At the same time, the full CDS length of the pectinase gene CgPG21 was cloned, encoding a protein composed of 480 amino acids. CgPG21 is mainly located in the cell wall, participates in the degradation of the intercellular layer of the cell wall during the development of the secretory cavity, and plays an important role in the formation of the secretory cavity in the intercellular space-forming and lumen-expanding stages. With the development of secretory cavity, the cell wall polysaccharides of epithelial cells gradually degrade. CgPG21 is mainly involved in the intercellular layer degradation.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Amelunxen F, Arbeiter H (1967) Untersuchungen an den Spritzdrusen von Dictamnus albus L. Zeitschrift Pflanzenphysiologie 58:49–69
Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev of Plant Biol 64(1):747–779
Babu Y, Bayer M (2014) Plant polygalacturonases involved in cell elongation and separation-the same but different? Plants 3(4):613–623
Bacic A, Stone BA (1981) Isolation and ultrastructure of aleurone cell walls from wheat and barley. Aust J Plant Physiol 8:453–474
Bai M, Liang M, Huai B, Gao H, Tong P, Shen R, He H, Wu H (2020) Ca2+-dependent nuclease is involved in DNA degradation during the programmed cell death of secretory cavity formation in Citrus grandis ‘Tomentosa’ fruits. J Exp Bot 71(16):4812–4827
Benarie R, Kislev N, Frenkel C (1979) Ultrastructural changes in the cell walls of ripening apple and pear fruit. Plant Physiol 64(2):197–202
Bennici A, Tani C (2004) Anatomical and ultrastructural study of the secretory cavity development of Citrus sinensis and Citrus limon: evaluation of schizolysigenous ontogeny. Flora 199(6):464–475
Bortiri E, Chuck G, Vollbrecht E, Rocheford T, Martienssen R, Hake S (2006) ramosa2 encodes alateral organ boundary domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18:574–585
Bosabalidis A, Tsekos I (1982a) Ultrastructural studies on the secretory cavities of Citrus deliciosa ten. I. Early stages of the gland cells differentiation. Protoplasma 112:55–62
Bosabalidis A, Tsekos I (1982b) Ultrastructural studies on the secretory cavities of Citrus deliciosa ten. II. Early stages of the gland cells differentiation. Protoplasma 112:63–70
Brocheriou J, Belin-Depoux M (1974) Contribution a I etude ontogenique des poches des feuilles de quelques Myrtaceae. Phytumurphology 24:321–338
Buat R (1989) Ontogeny, cell differentiation and structure of vascular plants. Springer-Verlag, Berlin
Chen Y, Wu H (2010) Programmed cell death involved in the schizolysigenous formation of the secretory cavity in Citrus sinensis (L) Osbeck. Chin Sci Bull 55(20):2160–2168
Cheng C, Zhang L, Yang X, Yang X, Zhong G (2015) Profiling gene expression in citrus fruit calyx abscission zone (AZ-C) treated with ethylene. Mol Genet Genomics 290(5):1991–2006
Cosgrove DJ (2018) Diffuse growth of plant cell walls. Plant Physiol 176(1):16–27
Daher FB, Braybrook SA (2015) How to let go: pectin and plant cell adhesion. Front in Plant Sci 6:523
Domozych DS, Sorensen I, Popper ZA, Ochs J, Andreas A, Fangel JU, Pielach A, Sacks C, Brechka H, Ruisi-Besares P, Willats W, Rose J (2014) Pectin metabolism and assembly in the cell wall of the charophyte green alga penium margaritaceum. Plant Physiol 165(1):105–118
Fabi JP, Broetto SG, da Silva SL, Zhong S, Lajolo FM (2014) Nascimento J (2014) Analysis of papaya cell wall-related genes during fruit ripening indicates a central role of polygalacturonases during pulp softening. PLoS ONE 9:e105685
Fohn M (1935) Zurentstehunq und weiterbildlung der exkreträume von Citrus medica L. und Eucalyptus globulus Lab. Österreichische Botanische Zeitschrift 84(3):198–209
Gapper NE, McQuinn RP, Giovannoni JJ (2013) Molecularandgenetic regulation of fruit ripening. Plant Mol Biol 82:575–591
Garg G, Singh A, Kaur A, Mahajan R (2016) Microbial pectinases: an ecofriendly tool of nature for Industries. Biotechnol 6:47
Gunawardena A, Pearce D, Jackson MB, Hawes CR, Evans DE (2001) Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize promoted by ethylene. Plant Cell Environ 24:1369–1375
Gunawardena A, Greenwood JS, Dengler NG (2007) Cell wall degradation and modification during programmed cell death in lace plant, Aponogeton madagascariensis (Aponogetonaceae). Am J Bot 94:1116–1128
Haberlandt G (1914) Physioloqical plant anotomy. London: Macmillan Press 777
He H, Bai M, Tong P, Yang M, Wu H (2018) Cellulase6 and mannanase7 affect cell differentiation and silique dehiscence. Plant Physiol 176(3):2186–2201
Hernrich G (1970) Elektronenmikroskopische beobachtungen an den drüsenzellen von Poncirus trifoliata; zugleich ein beitrag zur wirkung ätherischeröle auf pflanzenzellen und eine method zur unterscheidung flüchtiger von nichtflüchtigen lipophilen komponenten. Protoplasma 69(1):15–36
Huai B, Bai M, Tong P, He H, Liang M, Chen G, Wu H (2021) CgPBA1 may be involved in nuclear degradation during secretory cavity formation by programmed cell death in Citrus grandis ‘Tomentosa’ fruits. Plant Physiol Bioch 160:306–314
Kim J, Shiu S, Thoma S, Li WH, Patterson S (2006) Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol 7(9):1–14
Kisse JG (1958) Die Ausscheidung von atherischen glen und harzen. Handbuch Der Pflanzenphysiologie 10:91–131
Knight TG, Klieber A, Sedgley M (2001) The relationship between oil gland and fruit development in Washington navel orange (Citrus sinensis L Osbeck). Ann Bot-London. 88(6):1039–1047
Knight TG, Klieber A, Sedgley M (2002) Structural basis of the rind disorder oleocellosis in Washington navel orange (Citrus sinensis L Osbeck). Ann Bot-London. 90(6):765–773
Li C, Zhao M, Ma X, Wen Z, Ying P, Peng M, Ning X, Xia R, Wu H, Li J (2019) The HD-Zip transcription factor LcHB2 regulates litchi fruit abscission through the activation of two cellulase genes. J Exp Bot 70(19):5189–5203
Liang S, Wu H, Lun X, Lu D (2006) Secretory cavity development and its relationship with the accumulation of essential oil in fruits of Citrus medica L var sarcodactylis (Noot) Swingle. J Integr Plant Biol 48(5):573–583
Liang S, Wang H, Yang M, Wu H (2009) Sequential actions of pectinases and cellulases during secretory cavity formation in Citrus fruits. Trees 23(1):19–27
Liang M, Bai M, Wu H (2021) Zn2+-Dependent Nuclease IsInvolved in Nuclear Degradationduring the Programmed Cell Death ofSecretory Cavity Formation in Citrus grandis ‘Tomentosa’ Fruits. Cells 10:3222. https://doi.org/10.3390/cells10113222
Lin Y, Lin Y, Lin H, Lin M, Li H, Yuan F, Chen Y, Xiao J (2018) Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chem 264:1–4
Liu P, Liang S, Yao N, Wu H (2012) Programmed cell death of secretory cavity cells in fruits of Citrus grandis cv Tomentosa is associated with activation of caspase 3-like protease. Trees 26(6):1821–1835
Martinet J (1872) Organes de secretion des vegetaux. Annales Des Sci Nat Botan 14(5):91–232
Merelo P, Agusti J, Arbona V, Costa M, Estornell L, Gómez-Cadenas A, Coimbra S, Gómez M, Pérez-Amador M, Domingo C, Talón M, Tadeo F (2017) Cell wall remodeling in abscission zone cells during ethylene-promoted fruit abscission in Citrus. Front Plant Sci 8:126
Micheli F (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends in Plant Sci 6(9):414–419
Obara K, Fukuda H (2004) Programmed cell death in xylem differentiation. Programmed cell death in plants, Oxford, Blackwell
Peng Z, Liu G, Li H, Wang Y, Gao H, Jemrić T, Fu D (2022) Molecular and genetic events determining the softening of fleshy fruits: a comprehensive review. Int J Mol Sci 23(20):12482
Ram SS, Reeta RS, Ashok P, Christian L (2019) Advances in Enzyme Technology, Microbial Enzymes—An Overview. Elsevier, London, pp 1–40
Ren Y, Sun P, Wang X, Zhu Z (2020) Degradation of cell wall polysaccharides and change of related enzyme activities with fruit softening in Annona squamosa during storage. Postharvest Biol Tec 166:111203
Sieck W (1895) Die Schizolysigenen Secretbehalter. Jahrb Wiss Botanik 27:197–242
Sprecher E (1956) BeitrRge zur frage der biogenese sekundirer pflanzenstoffe der weinraute (Ruta graveolens L). Planta 47(4):323–358
Tariq A, Latif Z (2012) Isolation and biochemical characterization of bacterial isolates producing different levels of polygalacturonases from various sources. Afr J Microbiol Res 6(45):7259–7264
Thomson WW, Platt-Aloia KA, Endress AG (1967) Ultrastructure of oil gland development in the leaf of Citrus sinensis L. Bot Gaz 137:330–340
Tong P, Huai B, Chen Y, Bai M, Wu H (2020) CisPG21 and CisCEL16 are involved in the regulation of the degradation of cell walls during secretory cavity cell programmed cell death in the fruits of Citrus sinensis (L) Osbeck. Plant Sci 297:110540
Tschirch A, Stock E (1933) Die harze und die harzbehälter. Gebrüder Borntraeger, Beilin
Turner GW, Berry AM, Gifford EM (1998) Schizogenous secretory cavities of Citrus limon (L.) Burm. F. and a reevaluation of the lysigenous gland concept. Int J Plant Sci 159:75–88
Van Tieqhem P (1885) Deuxieme memoire sur les canaux secreteurs. Annales Des Sci Nat Botanique. 22:1–96
Wolf SI, Mouille G, Pelloux J (2009) Homogalacturonan methyl-esterification and plant development. Mol Plant 2(5):851–860
Wu Z, Li H, Yang Y, Zhan Y, Tu D (2013) Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind Crop Prod 46:311–316
Zamil MS, Geitmann A (2017) The middle lamella-more than a glu. Physical Biol 14(1):015004
Zhang B, Gao Y, Zhang L, Zhou Y (2021) The plant cell wall: Biosynthesis, construction, and functions. J Integr Plant Biol 63(1):251–272
Zheng P, Bai M, Chen Y, Liu P, Gao L, Liang S, Wu H (2014) Programmed cell death of secretory cavity cells of citrus fruits is associated with Ca2+ accumulation in the nucleus. Trees 28(4):1137–1144
Acknowledgements
This work was supported by the National Natural Science Foundation of China (project no. 32270381 to MB, 31870172 to HW), Natural Science Foundation of Guangdong (project no. 2022A1515011086 to MB), the Open Competition Program of Ten Major Directions of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (project no. 2022SDZG07 to HW), Laboratory of Lingnan Modern Agriculture Project (project no. NZ 2021024 to HW), Key Realm R&D Program of Guangdong Province (project no. 2020B020221001 to HW), Agricultural Science and Technology Innovation and Promotion Project of Guangdong (project no. 2018LM2160 to HW), and Research Fund of Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture (No. 2022KF009).
Funding
This work was supported by the National Natural Science Foundation of China (project no. 32270381 to MB), Natural Science Foundation of Guangdong (project no. 2022A1515011086 to MB), National Natural Science Foundation of China (project no. 31870172 to HW), the Open Competition Program of Ten Major Directions of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province, (project no. 2022SDZG07 to HW), Laboratory of Lingnan Modern Agriculture Project (project no. NZ 2021024 to HW), Key Realm R&D Program of Guangdong Province (project no. 2020B020221001 to HW), Agricultural Science and Technology Innovation and Promotion Project of Guangdong (project no. 2018LM2160 to HW), Research Fund of Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture (No. 2022KF009 to MB).
Author information
Authors and Affiliations
Contributions
HW and MB conceived the project. HW and MB designed the experiments. PT, NS, HH and BH performed the experiments and the data analysis. MB and HW wrote the manuscript, QL revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Additional information
Communicated by Leandro Peña.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai, M., Tong, P., Luo, Q. et al. CgPG21 is involved in the degradation of the cell wall during the secretory cavity formation in Citrus grandis ‘Tomentosa’ fruits. Plant Cell Rep 42, 1311–1331 (2023). https://doi.org/10.1007/s00299-023-03032-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-023-03032-7