Abstract
Key message
The presence of homologous subgenomes inhibited unreduced gamete formation in wheat × Aegilops interspecific hybrids. Unreduced gamete rates were under the control of the wheat nuclear genome.
Abstract
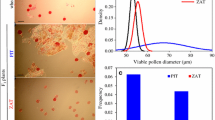
Production of unreduced gametes is common among interspecific hybrids, and may be affected by parental genotypes and genomic similarity. In the present study, five cultivars of Triticum aestivum and two tetraploid Aegilops species (i.e. Ae. triuncialis and Ae. cylindrica) were reciprocally crossed to produce 20 interspecific hybrid combinations. These hybrids comprised two different types: T. aestivum × Aegilops triuncialis; 2n = ABDUtCt (which lack a common subgenome) and T. aestivum × Ae. cylindrica; 2n = ABDDcCc (which share a common subgenome). The frequency of unreduced gametes in F1 hybrids was estimated in sporads from the frequency of dyads, and the frequency of viable pollen, germinated pollen and seed set were recorded. Different meiotic abnormalities recorded in the hybrids included precocious chromosome migration to the poles at metaphase I and II, laggards in anaphase I and II, micronuclei and chromosome stickiness, failure in cell wall formation, premature cytokinesis and microspore fusion. The mean frequency of restitution meiosis was 10.1 %, and the mean frequency of unreduced viable pollen was 4.84 % in T. aestivum × Ae. triuncialis hybrids. By contrast, in T. aestivum × Ae. cylindrica hybrids no meiotic restitution was observed, and a low rate of viable gametes (0.3 %) was recorded. This study present evidence that high levels of homologous pairing between the D and Dc subgenomes may interfere with meiotic restitution and the formation of unreduced gametes. Variation in unreduced gamete production was also observed between T. aestivum × Ae. triuncialis hybrid plants, suggesting genetic control of this trait.







Similar content being viewed by others
References
Bartek M, Hodnett G, Burson B, Stelly D, Rooney W (2012) Pollen tube growth after intergeneric pollinations of iap-homozygous Sorghum. Crop Sci 52:1553–1560
Bastiaanssen HJM, van den Berg PMMM, Lindhout P, Jacobsen E, Ramanna MS (1998) Postmeiotic restitution in 2n egg formation of diploid potato. Heredity 81:20–27
Blanco A, Simeone R, Tanzarella OA, Greco B (1983) Morphology and chromosome pairing of a hybrid between Triticum durum Desf. and Haynaldia villosa (L.) Schur. Theor Appl Genet 64:333–337
Bretagnolle F, Thompson J (1995) Tansley review no. 78. Gametes with the stomatic chromosome number: mechanisms of their formation and role in the evolution of autopolypoid plants. New Phytol 129:1–22
Cai X, Xu SS, Zhu X (2010) Mechanism of haploidy-dependent unreductional meiotic cell division in polyploid wheat. Chromosoma 119:275–285
Cheng C, McComb J (1992) In vitro germination of wheat pollen on raffinose medium. New Phytol 120:459–462
de Storme N, Geelen D (2013) Sexual polyploidization in plants—cytological mechanisms and molecular regulation. New Phytol 198:670–684
de Storme N, Mason A (2015) Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr Plant Biol 1:10–33
d’Erfurth I, Cromer L, Jolivet S, Girard C, Horlow C, Sun Y, To JP, Berchowitz LE, Copenhaver GP, Mercier R (2010) The cyclin-A CYCA1; 2/TAM is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition. PLoS Genet 6:e1000989
Fukuda K, Sakamoto S (1992) Studies on unreduced gamete formation in hybrids between tetraploid wheats and Aegilops squarrosa L. Hereditas 116:253–255
Hao M, Luo J, Zeng D, Zhang L, Ning S, Yuan Z, Yan Z, Zhang H, Zheng Y, Feuillet C, Choulet F, Yen Y, Zhang L, Liu D (2014) QTug.sau-3B is a major quantitative trait locus for wheat hexaploidization. G3 (Bethesda) 4:1943–1953
Harlan JR (1975) On Ö. Winge and a prayer: the origins of polyploidy. Bot Rev 41:361–390
Islam AKMR, Shepherd KW (1980) Meiotic restitution in wheat barley hybrids. Chromosoma 68:252–261
Jauhar PP (2007) Meiotic restitution in wheat polyhaploids (amphihaploids): a potent evolutionary force. J Hered 98:188–193
Kihara H, Lilienfeld F (1949) A new synthesized 6x-wheat. Hereditas 35(S1):307–319
Kobel HR (1996) Allopolyploid speciation. In: Tinsley RC, Kobel HR (eds) The biology of Xenopus. Clarendon Press, Oxford, pp 391–401
Leitch A, Leitch I (2008) Genomic plasticity and the diversity of polyploid plants. Science 320:481–483
Lim KB, Ramanna MS, de Jong JH, Jacobsen E, van Tuyl JM (2001) Indeterminate meiotic restitution (IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor Appl Genet 103:219–230
Loureiro I, Escorial C, Garcıa-Baudin JM, Chueca MC (2009) Spontaneous wheat-Aegilops biuncialis, Ae. geniculata and Ae. triuncialis amphiploid production, a potential way of gene transference. Span J Agric Res 7:614–620
Maan SS, Sasakuma T (1977) Fertility of amphihaploids in Triticinae. J Hered 57:76–83
Mason AS, Pires JC (2015) Unreduced gametes: meiotic mishap or evolutionary mechanism? Trends Genet 31:5–10
Mason A, Nelson M, Yan G, Cowling W (2011) Production of viable male unreduced gametes in Brassica interspecific hybrids is genotype specific and stimulated by cold temperatures. BMC Plant Biol 11:103
Matsuoka Y, Nasuda S (2004) Durum wheat as candidate for the unknown female progenitor of bread wheat: an empirical study with a highly fertile F1 hybrid with Aegilops tauschii Coss. Theor Appl Genet 109:1710–1717
Matsuoka Y, Nasuda S, Ashida Y, Nitta M, Tsujimoto H, Takumi S, Kawahara T (2013) Genetic basis for spontaneous hybrid genome doubling during allopolyploid speciation of common wheat shown by natural variation analyses of the paternal species. PLoS One 8:e68310
Minitab (2010) Minitab 16 statistical software. Minitab Inc., State College
Mirzaghaderi G, Fathi N (2015) Unreduced gamete formation in wheat: Aegilops triuncialis interspecific hybrids leads to spontaneous complete and partial amphiploids. Euphytica 206:67–75
Nelson MN, Mason AS, Castello M-C, Thomson L, Yan G, Cowling WA (2009) Microspore culture preferentially selects unreduced (2n) gametes from an interspecific hybrid of Brassica napus L.× Brassica carinata Braun. Theor Appl Genet 119:497–505
Peloquin SJ, Boiteux LS, Carputo D (1999) Meiotic mutants in potato: valuable variants. Genetics 153:1493–1499
Peng Z-S, Yang J, Zheng G-C (2003) Cytomixis in pollen mother cells of new synthetic hexaploid amphidiploid (Aegilops tauschii × Triticum turgidum). Cytologia 68:335–340
Peterson R, Slovin JP, Chen C (2010) A simplified method for differential staining of aborted and non-aborted pollen grains. Int J Plant Biol 1:e13
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Risso-Pascotto C, Pagliarini MS, Valle CB (2006) Microsporogenesis in Brachiaria dictyoneura (Fig. & Fe Not.) Stapf (Poaceae: Paniceae). Genet Mol Res 5:837–845
Schmidt A, Schmid MW, Grossniklaus U (2015) Plant germline formation: common concepts and developmental flexibility in sexual and asexual reproduction. Development 142:229–241
Shamina N, Dorogova N, Goncharov N, Orlova A, Trunova S (1999) Abnormalities of spindle and cytokine behavior leading to the formation of meiotic restitution nuclei in intergeneric cereal hybrids. Cell Biol Int 23:863–870
Silkova OG, Shchapova AI, Kravtsova LA (2003) Mechanisms of meiotic restitution and their genetic regulation in wheat–rye polyhaploids. Russ J Genet 39:1271–1280
Silkova O, Shchapova A, Shumny V (2011a) Meiotic restitution in amphihaploids in the tribe Triticeae. Russ J Genet 47:383–393
Silkova OG, Shchapova AI, Shumny VK (2011b) Patterns of meiosis in ABDR amphihaploids depend on the specific type of univalent chromosome division. Euphytica 178:415–426
Stefani A (1986) Unreduced gametes in the F1 hybrid of Triticum durum Desf. × Haynaldia villosa Schur. Zeitschrift für Pflanzenzüchtung 96:8–14
Tayalé A, Parisod C (2013) Natural pathways to polyploidy in plants and consequences for genome reorganization. Cytogenet Genome Res 140:79–96
Tiwari VK, Rawat N, Neelam K, Randhawa GS, Singh K, Chhuneja P, Dhaliwal HS (2008) Development of Triticum turgidum subsp. durum–Aegilops longissima amphiploids with high iron and zinc content through unreduced gamete formation in F1 hybrids. Genome 51:757–766
Utsunomiya KS, Pagliarini MS, Valle CBd (2004) Chromosome transfer among meiocytes in Brachiaria nigropedata (Ficalho & Hiern) Stapf (Gramineae). Cytologia 69:395–398
Wang C-J, Zhang L-Q, Dai S-F, Zheng Y-L, Zhang H-G, Liu D-C (2010) Formation of unreduced gametes is impeded by homologous chromosome pairing in tetraploid Triticum turgidum × Aegilops tauschii hybrids. Euphytica 175:323–329
Werner JE, Peloquin SJ (1991) Occurrence and mechanisms of 2n egg formation in 2x potato. Genome 34:975–982
Xu S, Dong Y (1992) Fertility and meiotic mechanisms of hybrids between chromosome autoduplication tetraploid wheats and Aegilops species. Genome 35:379–384
Xu S, Joppa L (1995) Mechanisms and inheritance of first division restitution in hybrids of wheat, rye, and Aegilops squarrosa. Genome 38:607–615
Xu S, Joppa L (2000) First-division restitution in hybrids of Langdon durum disomic substitution lines with rye and Aegilops squarrosa. Plant Breeding 119:233–241
Younis A, Hwang Y-J, Lim K-B (2014) Exploitation of induced 2n-gametes for plant breeding. Plant Cell Rep 33:215–223
Zeng D-Y, Hao M, Luo J-T, Zhang L-Q, Yuan Z-W, Ning S-Z, Zheng Y-L, Liu D-C (2014) Amphitelic orientation of centromeres at metaphase I is an important feature for univalent-dependent meiotic nonreduction. J Genet 93:531–534
Zhang L-Q, Yen Y, Zheng Y-L, Liu D-C (2007) Meiotic restriction in emmer wheat is controlled by one or more nuclear genes that continue to function in derived lines. Sex Plant Reprod 20:159–166
Zhang L-Q, Liu D-C, Zheng Y-L, Yan Z-H, Dai S-F, Li Y-F, Jiang Q, Ye Y-Q, Yen Y (2010) Frequent occurrence of unreduced gametes in Triticum turgidum–Aegilops tauschii hybrids. Euphytica 172:285–294
Acknowledgments
This research was financially supported by the University of Kurdistan, Sanandaj, Iran. ASM is funded by an Emmy Noether DFG award (MA 6473/1-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by I. Hwang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fakhri, Z., Mirzaghaderi, G., Ahmadian, S. et al. Unreduced gamete formation in wheat × Aegilops spp. hybrids is genotype specific and prevented by shared homologous subgenomes. Plant Cell Rep 35, 1143–1154 (2016). https://doi.org/10.1007/s00299-016-1951-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1951-9