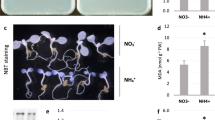
Abstract
Plants produce an immense number of natural products and undifferentiated cells from various plant tissues have long been considered an ideal source for their synthesis. However, undifferentiated plant cells often either lose their biosynthetic capacity over time or exhibit immediate repression of the required pathways once dedifferentiated. In this study, freshly prepared callus tissue was employed to further investigate the regulation of a natural product pathway in undifferentiated tobacco cells. Putrescine N-methyltransferase (PMT) is a pathway-specific enzyme required in nicotinic alkaloid production in Nicotiana species. Callus derived from transgenic Nicotiana tabacum plants harboring PMT promoter–GUS fusions were used to study factors that influence PMT expression. Under normal callus growth conditions in the presence of light and auxin, PMT promoter activity was strongly repressed. Conversely, dark conditions and the absence of auxin were found to upregulate PMT promoter activity, with light being dominant to the repressive effects of auxin. Since reactive oxygen species (ROS) are known by-products of photosynthesis and have been implicated in signaling, their involvement was investigated in transgenic callus by treatment with the ROS scavenger, dimethylthiourea, or catalase. Under highly repressive conditions for alkaloid synthesis, including normal culture conditions in the light, both ROS scavengers resulted in significant induction of PMT promoter activity. Moreover, treatment of callus with catalase resulted in the upregulation of PMT promoter activity and alkaloid accumulation in this tissue. These results suggest that ROS impact the regulation of the alkaloid pathway in undifferentiated cells and have implications for regulation of the pathway in other plant tissues.





Similar content being viewed by others
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Baldwin IT (1989) Mechanism of damage-induced alkaloid production in wild tobacco. J Chem Ecol 15:1661–1680
Baldwin IT (1996) Allometric limits to the induced accumulation of nicotine in native tobacco. Plant Species Biol 11:107–114
Baldwin IT (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. PNAS 95:8113–8118
Baldwin IT, Hamilton W III (2000) Jasmonate-induced responses of Nicotiana sylvestris results in fitness costs due to impaired competitive ability for nitrogen. J Chem Ecol 26:915–952
Baldwin IT, Schmelz EA, Ohnmeiss TE (1994) Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris spegazzini and comes. J Chem Ecol 20:2139–2157
Baldwin IT, Schmelz EA, Zhang Z-P (1996) Effects of octadecanoid metabolites and inhibitors on induced nicotine accumulation in Nicotiana sylvestris. J Chem Ecol 22:61–74
Baldwin IT, Zhang Z-P, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA (1997) Quantification, correlations and manipulation of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201:397–404
Baldwin IT, Gorham D, Schmelz EA, Lewandowski CA, Lynds GY (1998) Allocation of nitrogen to an inducible defense and seed production in Nicotiana attenuata. Oecologia (Berlin) 115:541–552
Bartholomeusz TA, Bhogal RK, Molinie R, Felpin FX, Mathe-Allainmat M, Meier AC, Drager B, Lebreton J, Roscher A, Robins RJ, Mesnard F (2005) Nicotine demethylation in Nicotiana cell suspension cultures: N′-formylnornicotine is not involved. Phytochemistry 66:2432–2440
Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P, Dixon RA, Sumner LW (2005) Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J Exp Bot 56:323–336
Casano LM, Martin M, Sabater B (2001) Hydrogen peroxide mediates the induction of chloroplastic Ndh complex under photooxidative stress in barley. Plant Physiol 125:1450–1458
Chintapakorn Y, Hamill JD (2003) Antisense-mediated down-regulation of putrescine N-methyltransferase activity in transgenic Nicotiana tabacum L. can lead to elevated levels of anatabine at the expense of nicotine. Plant Mol Biol 53:87–105
De Sutter V, Vanderhaeghen R, Tilleman S, Lammertyn F, Vanhoutte I, Karimi M, Inze D, Goossens A, Hilson P (2005) Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant J 44:1065–1076
Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330(Pt 1):115–120
Feth F, Wagner R, Wagner KG (1986) Regulation in tobacco callus of enzyme activities of the nicotine pathway. I. The route ornithine to methylpyrroline. Planta 168:402–407
Finkel T (1998) Oxygen radicals and signaling. Curt Opin Cell Biol 10:248–253
Fritze K, Walden R (1995) Gene activation by T-DNA tagging. In: Methods in molecular biology. Humana Press, Clifton, pp 281–294
Gattu M, Terry AV Jr, Buccafusco JJ (1995) A rapid microtechnique for the estimation of muscarinic and nicotinic receptor binding parameters using 96-well filtration plates. J Neurosci Methods 63:121–125
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28:1091–1101
George J, Bais HP, Ravishankar GA (2000) Biotechnological production of plant-based Insecticides. Crit Rev Biotechnol 20:49–77
Goossens A, Häkkinen ST, Laakso I, Seppanen-Laakso T, Biondi S, De Sutter V, Lammertyn F, Nuutila AM, Saderlund H, Zabeau M, Inze’ D, Oksman-Caldentey K-M (2003) A functional genomics approach toward the understanding of secondary metabolism in plant cells. PNAS 100:8595–8600
Guo ZJ, Lamb C, Dixon RA (1998) Potentiation of the oxidative burst and isoflavonoid phytoalexin accumulation by serine protease inhibitors. Plant Physiol 118:1487–1494
Häkkinen ST, Tilleman S, Swiatek A, De Sutter V, Rischer H, Vanhoutte I, Van Onckelen H, Hilson P, Inzé D, Oksman-Caldentey K-M, Goossens A (2007) Functional characterisation of genes involved in pyridine alkaloid biosynthesis in tobacco. Phytochemistry 68:2773–2785
Hashimoto T, Yamada Y (1994) Alkaloid biogenesis: molecular aspects. Ann Rev Plant Physiol Plant Mol Biol 45:257–285
Hashimoto T, Tamaki K, Suzuki K, Yamada Y (1998) Molecular cloning of plant spermidine synthases. Plant Cell Physiol 39:73–79
He Y, Gan S (2001) Identical promoter elements are involved in regulation of the OPR1 gene by senescence and jasmonic acid in Arabidopsis. Plant Mol Biol 47:595–605
Henzler T, Steudle E (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot 51:2053–2066
Hibi N, Higashiguchi S, Hashimoto T, Yamada Y (1994) Gene expression in tobacco low-nicotine mutants. Plant Cell 6:723–735
Hobbs MC, Yeoman MM (1991a) Biotransformation of nicotine to nornicotine by cell suspensions of Nicotiana tabacum l cv wisconsin-38. New Phytol 119:477–482
Hobbs MC, Yeoman MM (1991b) Effect of light on alkaloid accumulation in cell cultures of Nicotiana spp. J Exp Bot 42:1371–1378
Houghtling RA, Davila-Garcia MI, Kellar KJ (1995) Characterization of (±)(-)[3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Mol Pharmacol 48:280–287
Imanishi S, Hashizume K, Nakakita M, Kojima H, Matsubayashi Y, Hashimoto T, Sakagami Y, Yamada Y, Nakamura K (1998) Differential induction by methyl jasmonate of genes encoding ornithine decarboxylase and other enzymes involved in nicotine biosynthesis in tobacco cell cultures. Plant Mol Biol 38:1101–1111
Iwase A, Aoyagi H, Ohme-Takagi M, Tanaka H (2005) Development of a novel system for producing ajmalicine and serpentine using direct culture of leaves in Catharanthus roseus intact plant. J Biosci Bioeng 99:208–215
Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D (1997) Elicitor-stimulated ion fluxes and O2 − from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. PNAS 94:4800–4805
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284:654–657
Kidd SK, Melillo AA, Lu RH, Reed DG, Kuno N, Uchida K, Furuya M, Jelesko JG (2006) The A and B loci in tobacco regulate a network of stress response genes, few of which are associated with nicotine biosynthesis. Plant Mol Biol 60:699–716
Kroj T, Rudd JJ, Nurnberger T, Gabler Y, Lee J, Scheel D (2003) Mitogen-activated protein kinases play an essential role in oxidative burst-independent expression of pathogenesis-related genes in parsley. J Biol Chem 278:2256–2264
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Littleton J, Rogers T, Falcone D (2005) Novel approaches to plant drug discovery based on high throughput pharmacological screening and genetic manipulation. Life Sci 78:467–475
Maliga P, Sz-Breznovits A, Marton L (1973) Streptomycin resistant plants from callus culture of haploid tobacco. Nat New Biol 244:29–30
Mehdy MC (1994) Active oxygen species in plant defense against pathogens. Plant Physiol 105:467–472
Mizusaki S, Tanebe Y, Noguchi M, Tamaki E (1973) Changes in activities of ornithine decarboxylase, putrescine N-methyltransferase and N-methylputrescine oxidase in tobacco roots in relation to nicotine biosynthesis. Plant Cell Physiol 14:103–110
Moyano E, Fornale S, Palazon J, Cusido RM, Bagni N, Pinol MT (2002) Alkaloid production in Duboisia hybrid hairy root cultures overexpressing the pmt gene. Phytochemistry 59:697–702
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247
O’Kane D, Gill V, Boyd P, Burdon R (1996) Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta 198:371–377
Ogino T, Hiraoka N, Tabata M (1978) Selection of high nicotine producing cell lines of tobacco callus by single cell cloning. Phytochemistry 17:1907–1910
Ohnmeiss TE, McCloud ES, Lynds GL, Baldwin IT (1997) Within-plant relationships among wounding, jasmonic acid, and nicotine: Implications for defence in Nicotiana sylvestris. New Phytol 137:441–452
Ohta S, Matsui O, Yatazawa M (1978) Culture conditions for nicotine production in tobacco tissue culture. Agric Biol Chem 42:1245–1252
Pfeiffer W, Hoeftberger M (2001) Oxidative burst in Chenopodium rubrum suspension cells: Induction by auxin and osmotic changes. Physiol Plant 111:144–150
Phillips I (1975) Apical dominance. Annu Rev Plant Physiol 26:341–365
Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT (1999) Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta 209:87–95
Riechers DE, Timko MP (1999) Structure and expression of the gene family encoding putrescine N-methyltransferase in Nicotiana tabacum: new clues to the evolutionary origin of cultivated tobacco. Plant Mol Biol 41:387–401
Rogers DT, Falcone DL, Littleton JM (2003) A functional genomics strategy for plant drug discovery. Pharmagenomics 3:26–34
Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, Whelan J, Kazan K, Pogson BJ (2007) Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19:4091–4110
Rothe G, Hachiya A, Yamada Y, Hashimoto T, Drager B (2003) Alkaloids in plants and root cultures of Atropa belladonna overexpressing putrescine N-methyltransferase. J Exp Bot 54:2065–2070
Sachan N, Falcone DL (2002) Wound-induced gene expression of putrescine N-methyltransferase in leaves of Nicotiana tabacum. Phytochemistry 61:797–805
Saedler R, Baldwin IT (2004) Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. J Exp Bot 55:151–157
Sato F, Hashimoto T, Hachiya A, Tamura K, Choi KB, Morishige T, Fujimoto H, Yamada Y (2001) Metabolic engineering of plant alkaloid biosynthesis. PNAS 98:367–372
Saunders JW, Bush LP (1979) Nicotine biosynthetic enzyme activities in Nicotiana tabacum L. genotypes with different alkaloid levels. Plant Physiol 64:236–240
Shoji T, Hashimoto T (2008) Why does snatabine, but not nicotine, accumulate in jasmonate-elicited cultured tobacco BY-2 cells? Plant Cell Physiol 49:1209–1216
Shoji T, Yamada Y, Hashimoto T (2000) Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant Cell Physiol 41:831–839
Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT (2004) Nicotine’s defensive function in nature. PLoS Biol 2:E217
Suzuki K, Yamada Y, Hashimoto T (1999) Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol 40:289–297
Tabata M, Hiraoka N (1976) Variation of alkaloid production in Nicotiana rustica var–Brasilia callus cultures. Physiol Plant 38:19–23
Tabata M, Yamamoto H, Hiraoka N, Marumoto Y, Konoshima M (1971) Regulation of nicotine production in tobacco-d tissue culture by plant growth regulators. Phytochemistry 10:723–729
Takahashi M, Yamada Y (1973) Regulation of nicotine production by auxins in tobacco cultured cells in vitro. Agric Biol Chem 37:1755–1757
Tiburcio AF, Kaur-Sawhney R, Ingersoll RB, Galston AW (1985) Correlation between polyamines and pyrrolidine alkaloids in developing tobacco callus. Plant Physiol 78:323–326
Voelckel C, Kruegel T, Gase K, Heidrich N, van Dam NM, Winz R, Baldwin IT (2001) Anti-sense expression of putrescine N-methyltransferase confirms defensive role of nicotine in Nicotiana sylvestris against Manduca sexta. Chemoecology 11:121–126
Wink M (1998) Modes of action of alkaloids. In: Roberts M, Wink M (eds) Alkaloids. Plenum Press, New York, pp 301–326
Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation : evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83:278–282
Wu J, Ge X (2004) Oxidative burst, jasmonic acid biosynthesis, and taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol Bioeng 85:714–721
Xu B, Timko M (2004) Methyl jasmonate induced expression of the tobacco putrescine N-methyltransferase genes requires both G-box and GCC-motif elements. Plant Mol Biol 55:743–761
Yin S, Mei L, Newman J, Back K, Chappell J (1997) Regulation of sesquiterpene cyclase gene expression. Characterization of an elicitor- and pathogen-inducible promoter. Plant Physiol 115:437–451
Zhang Z-P, Krumm T, Baldwin IT (1997) Structural requirements of jasmonates and mimics for nicotine induction in Nicotiana sylvestris. J Chem Ecol 23:2777–2789
Zhao J, Sakai K (2003) Multiple signaling pathways mediate fungal elicitor induced b-thujaplicin accumulation in Cupressus lusitanica cell cultures. J Exp Bot 54:647–656
Zhao J, Fujita K, Sakai K (2005) Oxidative stress in plant cell culture: a role in production of beta-thujaplicin by Cupresssus lusitanica suspension culture. Biotechnol Bioeng 90:621–631
Zhao J, Matsunaga Y, Fujita K, Sakai K (2006) Signal transduction and metabolic flux of beta-thujaplicin and monoterpene biosynthesis in elicited Cupressus lusitanica cell cultures. Metab Eng 8:14–29
Acknowledgments
We wish to thank Irina Artiushin and May Fu for expert technical assistance with protoplast preparations and alkaloid binding assays, respectively. This work was supported by the Kentucky Tobacco Research Board and the University of Massachusetts Lowell.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Somers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sachan, N., Rogers, D.T., Yun, KY. et al. Reactive oxygen species regulate alkaloid metabolism in undifferentiated N. tabacum cells. Plant Cell Rep 29, 437–448 (2010). https://doi.org/10.1007/s00299-010-0833-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0833-9