Abstract
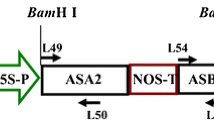
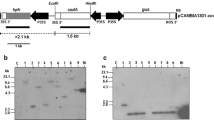
Effective selectable markers are needed for basic research and commercial applications that do not involve antibiotic or herbicide resistance. A novel selection system based on a feedback-insensitive anthranilate synthase α-subunit of tobacco (ASA2) as selectable marker using either 4-methylindole (4MI) or 7-methyl-DL-tryptophan (7MT) as the selection agent was developed. We found that these two components were able to discriminate better between ASA2 expressing and untransformed lines than the most commonly used analog 5-methyltryptopan (5MT) in the seedling growth inhibition test. We successfully integrated an expression cassette containing an ASA2 cDNA driven by a cauliflower mosaic virus 35S promoter into tobacco leaf discs by A. tumefaciens and selected transgenic plants on medium supplemented with 300 μM of 7MT or 4MI. Due to the expression of the feedback-insensitive ASA2, the transgenic lines produced showed higher free tryptophan (Trp) concentrations than the untransformed WT control. These results demonstrate the feasibility of the selection system with the ASA2 gene in combination with the use of Trp or indole analogs as selective agent.







Similar content being viewed by others
Abbreviations
- AS:
-
Anthranilate synthase
- BA:
-
6-Benzyladenine
- NAA:
-
Naphthalene acetic acid
- Trp:
-
Tryptophan
- 7MT:
-
7-Methyl-DL-tryptophan
- 4MI:
-
4-Methylindole
References
Anderson PC, Chomet PS, Griffor MC, Kriz AL (1997) Anthranilate synthase gene and its use thereof. World Intellectual Property Organization 97/26366. World Intellectual Property Organization
Bernasconi P, Walters EW, Woodworth AR, Siehl DL, Stone TE, Subramanian MW (1994) Functional expression of Arabidopsis thaliana anthranilate synthase subunit I in Escherichia coli. Plant Physiol 106:353–358
Bohlmann J, Lins T, Martin W, Eilert U (1996) Anthranilate synthase from Ruta graveolens. Duplicated ASa genes encode tryptophan-sensitive and tryptophan-insensitive isoenzymes specific to amino acid and alkaloid biosynthesis. Plant Physiol 111:507–514
Carlson JE, Widholm JM (1978) Separation of two forms of anthranilate synthetase from 5-methyltryptophan-susceptible and -resistant cultured Solanum tuberosum cells. Physiol Plant 44:251–255
Cho HJ, Brotherton JE, Song H-S, Widholm JM (2000) Increasing tryptophan synthesis in a forage legume Astragalus sinicus by expressing the tobacco feedback-insensitive anthranilate synthase (ASA2) gene. Plant Physiol 123:1069–1076
Crawdord IP (1989) Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol 43:567–600
Fitter A, Perrins J, Williamson M (1990) Weed probability challenged. Biotechnology 8:473
Hanafy MS, Rahman SM, Khalafalla MM , El-Shemy HA , Nakamoto Y, Ishimoto M, Wakasa K (2006) Accumulation of free tryptophan in azuki bean (Vigna angularis) induced by expression of a gene (OASA1D) for a modified α-subunit of rice anthranilate synthase. Plant Science 171(6):670–676
Haslam E (1993) Shikim acid: metabolism and metabolites. Wiley, Chichester
Hohn B, Levy AA, Puchta H (2001) Elimination of selection markers from transgenic plants. Curr Opin Biotechnol 12:139–143
Hood EA, Gelvin SB, Melchers LS, Hoekema A (1993) New agrobacterium helper plasmids for gene transfer to plants. Transgen Res 2:208–218
Horsch RB, Rogers SG, Fraley RT (1985) Transgenic plants. Cold Spring Harb Symp Quant Biol 50:433–437
Inaba Y, Brotherton JE, Ulanov A, Widholm JM (2007) Expression of a feedback insensitive anthranilate synthase gene from tobacco increases free tryptophan in soybean plants. Plant Cell Rep (in press)
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Kreps JA, Town CD (1992) Isolation and characterization of a mutant of Arabidopsis thaliana resistant to alpha methyltryptophan. Plant Physiol 99:269–275
Leyman B, Avonce N, Ramon M, Van Dijck P, Thevelein JM, Iturriaga G (2004) New selection marker for plant transformation. Methods Mol Biol 267:385–396
Leyman B, Avonce N, Ramon M, Dijck PV, Inturriaga G, Thevelein JM (2005) Trehalose-6-phosphate synthase as an intrinsic selection marker for plant transformation. J Biotechnol 121(3):309–317
Li J, Last RL (1996) The Arabidopsis thaliana trp5 mutant has a feedback-resistant anthranilate synthase and elevated soluble tryptophan. Plant Physiol 110:51–59
Mentewab A, Stewart CN Jr (2005) Overexpression of an Arabidopsis thaliana ABC transporter confers kanamycin resistance to transgenic plants. Nat Biotechnol 23(9):1177–1180
Miki B, McHugh S (2004) Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotechnol 107(3):193−232
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Murray, Thompson (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321−4325
Poulsen C, Bongaerts RJM, Verpoorte R (1993) Purification and characterization of anthranilate synthase from Catharanthus roseus. Eur J Biochem 212:431–440
Radwanski ER, Last RL (1995) Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7:921–934
Ranch JP, Rick S, Brotherton JE, Widholm JM (1983) Expression of 5-methyltryptophan resistance in plants regenerated from resistant cell lines of Datura innoxia. Plant Physiol 71:136–140
Rieger M, Preston C, Powles S (1999) Risks of gene flow from transgenic herbicide-resistant canola (Brassica napus) to weedy relatives in southern Australian cropping systems. Aust J Agric Res 50:115–128
Romero RM, Roberts MF, Phillipson JD (1995) Anthranilate synthase in microorganisms and plants. Phytochemistry 39:263–276
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Singh M, Widholm JM (1974) Measurement of the five enzymes which convert chorismate to tryptophan in wheat plants (Triticum aestivum L.). Physiol Plant 32:362–366
Song H-S, Brotherton JE, Gonzales RA, Widholm JM (1998) Tissue culture specific expression of a feedback-insensitive Nicotiana tabacum anthranilate synthase. Plant Physiol 117:533–543
Tsai FY, Brotherton JE, Widholm JM (2004) Overexpression of the feedback-insensitive anthranilate synthase gene in tobacco causes tryptophan accumulation. Plant Cell Rep 23:548–556
Wakasa K, Widholm JM (1987) A 5-methyltryptophan resistant rice mutant, MTR1, selected in tissue culture. Theor Appl Genet 74:49–54
Widholm JM (1972) Cultured Nicotiana tabacum cells with an altered anthranilate synthase which is less sensitive to feedback inhibition. Biochim Biophys Acta 261:52–58
Widholm JM (1974) Control of aromatic amino acid biosynthesis in cultured plant tissues: effect of intermediates and aromatic amino acids on free levels. Physiol Plant 30:13–18
Widholm (1981) Utilization of indole analogs by carrot and tobacco cell tryptophan synthase in vivo and in vitro. Plant Physiol 67(6):1101–1104
Yamada T, Tozawa Y, Hasegawa H, Terakawa T, Ohkawa Y, Wakasa K (2004) Use of the feedback-insensitive α-subunit of anthranilate synthase as a selectable marker for transformation of rice and potato. Mol Breed 14:363–373
Zhang XH, Brotherton JE, Widholm JM, Portis AR (2001) Targeting a nuclear anthranilate synthase alpha-subunit gene to the tobacco plastid genome results in enhanced tryptophan biosynthesis. Return of a gene to its pre-endosymbiotic origin. Plant Phys 127(1):131–141
Acknowledgments
The authors thank Wei Q. Zhong and Guirong Zhang for their technical assistance and Dr. Anatoliy V. Lygin for tryptophan content measurement. This research was supported in part through The Consortium for Plant Biotechnology Research, Inc., by DOE Prime Agreement No.GO12026–175. This support does not constitute an endorsement by DOE or The Consortium for Plant Biotechnology Research, Inc., of the views expressed in this publication. This study was also supported in part by funds from the Illinois Agricultural Experiment Station, the Biotechnology Research and Development Corp. and Dow AgroSciences LLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Altman.
Rights and permissions
About this article
Cite this article
Barone, P., Widholm, J.M. Use of 4-methylindole or 7-methyl-DL-tryptophan in a transformant selection system based on the feedback-insensitive anthranilate synthase α-subunit of tobacco (ASA2). Plant Cell Rep 27, 509–517 (2008). https://doi.org/10.1007/s00299-007-0480-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0480-y