Abstract
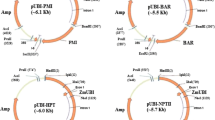
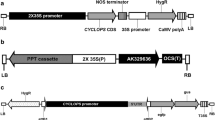
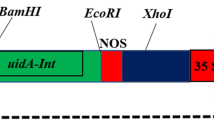
A protocol for Agrobacterium-mediated transformation with mannose selection was developed for cotyledon petiole, hypocotyl and leaf explants of tomato (Lycopersicon esculentum L. Mill). More than 400 transgenic plants from three tomato varieties were selected with 1% mannose in combination with 0.1–0.5% glucose. Average transformation frequencies ranged from 2.0 to 15.5% depending on the construct, genotype and type of tissue used for transformation. The highest transformation rate was obtained for hypocotyl explants from tomato variety SG048. The ploidy levels of 264 independent transgenic events and 233 non-transgenic plants regenerated from tissue culture were assessed by flow cytometry. The incidence of polyploids within the total population of transgenic plants varied from 10 to 78% and was not significantly different from the non-transgenic population. The greatest variation in the proportion of polyploids was observed in plants derived from different explant types, both in transgenic and non-transgenic regenerants, across three studied genotypes. Transgenic and non-transgenic plants regenerated from leaves included the highest number of normal diploid plants (82–100%), followed by cotyledon petiole-derived plants (63–78%). Transgenic plants produced from hypocotyls contained 22–58% diploids depending on the genotype used in transformation. Results described in this study demonstrate that, although transformation frequencies for leaf tissue are still lower under current protocols, the high percentage of diploids obtained make leaf tissue an attractive transformation target.






Similar content being viewed by others
Abbreviations
- BAP:
-
Benzylaminopurine
- MS:
-
Murashige-Skoog
- MsCHI:
-
Medicago sativa chalcone isomerase
- PMI:
-
Phosphomannose isomerase
References
Boscariol RL, Almeida WAB, Derbyshire MTVC, Mourao Filho FAA, Mendes BMJ (2003) The use of the PMI/mannose selection system to recover transgenic sweet orange plants (Citrus sinensis L. Osbeck). Plant Cell Rep 22:122–128
Bulk RW van den, Loffler HJM, Lindhout WH, Koornneef M (1990) Somaclonal variation in tomato: effect of explant source a comparison with chemical mutagenesis. Theor Appl Genet 80:817–825
Callis J, Raasch JA, Vierstra RD (1990) Ubiquitin extension proteins of Arabidopsis thaliana: structure, localization and expression of their promoters in transgenic tobacco. J Biol Chem 265:12486–12493
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Chyi YS, Phillips GC (1987) High efficiency Agrobacterium-mediated transformation of Lycopersicon based on conditions favorable for regeneration. Plant Cell Rep 6:105–108
Davis ME, Lineberger RD, Miller AR (1991) Effects of tomato cultivar, leaf age, and bacterial strain on transformation by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 24:115–121
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Ellul P, Garcia-Sogo B, Pineda B, Rios G, Roig LA, Moreno V (2003) The ploidy level of transgenic plants in Agrobacterium-mediated transformation of tomato cotyledons (Lycopersicon esculentum L. Mill.) is genotype and procedure dependent. Theor Appl Genet 106:231–238
Fillati JJ, Kiser J, Rose R, Comai L (1987) Efficient transfer of a glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Biotechnology 5:726–730
Fling ME, Kopf J, Richards C (1985) Nucleotide sequence of the transposon Tn7 gene encoding an aminoglycoside-modifying enzyme, 3″(9)-0-nucleotidyltransferase. Nucleic Acids Res 13:7095–7106
Frary A, Earle ED (1996) An examination of factors affecting the efficiency of Agrobacterium-mediated transformation of tomato. Plant Cell Rep 16:235–240
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:150–158
Haldrup A, Petersen SG, Okkels FT (1998) The xylose isomerase gene from Thermoanaerobacterium thermosulfurogenes allows effective selection of transgenic plant cells using d-xylose as the selection agent. Plant Mol Biol 37:287–296
Hamza S, Chupeau Y (1993) Re-evaluation of conditions for plant regeneration and Agrobacterium-mediated transformation from tomato (Lycopersicon esculentum). J Exp Bot 44:1837–1845
Hohn B, Levy AA, Puchta H (2001) Elimination of selection markers from transgenic plants. Curr Opin Biotechnol 12:139–143
Hu W, Phillips GC (2001) A combination of overgrowth-control antibiotics improves Agrobacterium tumefaciens-mediated transformation efficiency for cultivated tomato (L. esculentum). In Vitro Cell Dev Biol Plant 37:12–18
Ingham DJ, Beer S, Money S, Hansen G (2001) Quantitative real-time PCR assay for determining transgene copy number in transformed plants. BioTechniques 31:132–140
Itoh Y, Watson JM, Haas D, Leisinger T (1984) Genetic and molecular characterization of the Pseudomonas plasmid pVS1. Plasmid 11:206–220
Jacobs JP, Yoder JI (1989) Ploidy levels in transgenic plants determined by chloroplast number. Plant Cell Rep 7:662–664
Joersbo M, Donaldson I, Kreiberg J, Petersen SG, Brunstedt J, Okkels FT (1998) Analysis of mannose selection used for transformation of sugar beet. Mol Breed 4:111–117
Joersbo M, Petersen SG, Okkels FT (1999) Parameters interacting with mannose selection employed for the production of transgenic sugar beet. Physiol Plant 105:109–115
Joubes J, Chevalier C (2000) Endoreduplication in higher plants. Plant Mol Biol 43:735–745
Koornneef M, Hanhart C, Jongsma M, Toma I, Weide R, Zabel P, Hille J (1986) Breeding of a tomato genotype readily accessible to genetic manipulation. Plant Sci 45:201–208
Kudo N, Kimura Y (2001a) Flow cytometric evidence for endopolyploidy in seedlings of some Brassica species. Theor Appl Genet 102:104–110
Kudo N, Kimura Y (2001b) Patterns of endopolyploidy during seedling development in cabbage (Brassica oleracea L.). Ann Bot 87:275–281
Ling H-Q, Kriseleit D, Ganal MW (1998) Effect of ticarcillin/potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill.). Plant Cell Rep 17:843–847
Lucca P, Ye X, Potrykus I (2001) Effective selection and regeneration of transgenic rice plants with mannose as selective agent. Mol Breed 7:43–49
Maliga P (1982) Cell culture procedures for Nicotiana plumbaginifolia. Plant Mol Biol News 3:88–94
Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA (1999) Fluorescent proteins from non bioluminescent Anthozoa species. Nat Biotechnol 17:969–973
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep 5:81–85
Miles JS, Guest JR (1984) Nucleotide sequence and transcriptional start point of the phosphomannose isomerase gene (manA) of Escherichia coli. Gene 32:41–48
Mishiba K, Mii M (2000) Polysomaty analysis in diploid and tetraploid Portulaca grandiflora. Plant Sci 156:213–219
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Negrotto D, Jolley M, Beer S, Wenck AR, Hansen G (2000) The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep 19:798–803
Park SH, Morris JL, Park JE, Hirschi KD, Smith RH (2003) Efficient and genotype-independent Agrobacterium-mediated tomato transformation. J Plant Physiol 160:1253–1257
Penna S, Sagi L, Swennen R (2002) Positive selectable marker genes for routine plant transformation. In Vitro Cell Dev Biol Plant 38:125–128
Reed JN, Privalle LS, Powell MJ, Meghji M, Dawson J, Dunder EM, Suttie J, Wenck AR, Launis K, Kramer C, Chang Y-F, Hansen G, Wright M (2001) Phosphomannose isomerase: an efficient selectable marker for plant transformation. In Vitro Cell Dev Biol Plant 37:127–132
Roekel JSC van, Damm B, Melchers LS, Hoekema A (1993) Factors influencing transformation frequency of tomato (Lycopersicon esculentum). Plant Cell Rep 12:644–647
Shahin EA, Sukhapinda K, Simpson RB, Spivey R (1986) Transformation of cultivated tomato by a binary vector in Agrobacterium rhizogenes: transgenic plants with normal phenotypes harbor binary vector T-DNA, but not Ri-plasmid T-DNA. Theor Appl Genet 72:770–777
Smulders MJM, Rus-Kortekaas W, Gilissen LJW (1994) Development of polysomaty during differentiation in diploid and tetraploid tomato (Lycopersicon esculentum) plants. Plant Sci 97:53–60
Smulders MJM, Rus-Kortekaas W, Gilissen LJW (1995) Natural variation in patterns of polysomaty among individual tomato plants and their regenerated progeny. Plant Sci 106:129–139
Stavolone L, Kononova M, Pauli S, Ragozzino A, de Haan P, Milligan S, Lawton K, Hohn T (2003) Cestrum yellow leaf curling virus (CmYLCV) promoter: a new strong constitutive promoter for heterologous gene expression in a wide variety of crops. Plant Mol Biol 53:703–713
Ultzen T, Gielen J, Venema F, Westerbroek A, de Haan P, Tan M-L, Schram A, van Grinsven M, Goldbach R (1995) Resistance to tomato spotted wilt virus in transgenic tomato hybrids. Euphytica 85:159–168
Wang AS, Evans RA, Altendorf PR, Hanten JA, Doyle MC, Rosichan JL (2000) A mannose selection system for production of fertile transgenic maize plants from protoplasts. Plant Cell Rep 19:654–660
Wright M, Dawson J, Dunder E, Suttie J, Reed J, Kramer C, Chang Y, Novitzky R, Wang H, Artim-Moore L (2001) Efficient biolistic transformation of maize (Zea mays L.) and wheat (Triticum aestivum L.) using the phosphomannose isomerase gene, pmi, as the selectable marker. Plant Cell Rep 20:429–436
Zhang P, Puonti-Kaerlas J (2000) PIG-mediated cassava transformation using positive and negative selection. Plant Cell Rep 19:1041–1048
Acknowledgements
We are grateful to Natasha Kornegay for assisting with tomato transformation experiments, Yu-Yu Bai for helping with statistical analysis, Mary Fielder for performing the TaqMan assay and Brian Potter for taking care of plants in the greenhouse.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.D. Earle
Rights and permissions
About this article
Cite this article
Sigareva, M., Spivey, R., Willits, M.G. et al. An efficient mannose selection protocol for tomato that has no adverse effect on the ploidy level of transgenic plants. Plant Cell Rep 23, 236–245 (2004). https://doi.org/10.1007/s00299-004-0809-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0809-8