Abstract
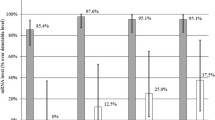
We examined whether the expression and activation of pro-matrix metalloproteinase (MMP)-1 varies from that of pro-MMP-13 in the joint fluid of osteoarthritis (OA) and rheumatoid arthritis (RA) patients. To do this, joint fluid was collected from 34 RA and 34 OA patients. The collagenase (pro-MMP-1 and MMP-13, total MMP-1, and MMP-13), gelatinase (total MMP-2 and MMP-9), stromelysin (total MMP-3), matrilysin (total MMP-7), uPA, and tissue inhibitor of MMP (TIMP) levels were measured by ELISA. The level of total MMP-1 in RA joint fluids was similar to that of the OA joint fluid. In contrast, the level of total MMP-13 in the RA group was significantly higher than that of the OA group. Among various MMPs (MMP-2, MMP-3, MMP-7, and MMP-9), only MMP-9 was strongly associated with total MMP-13 in both RA and OA. The level of uPA was also strongly associated with MMP-13 in RA but not OA, while the level of TIMP-1 and TIMP-2 was not significantly different between RA and OA. In conclusion, MMP-9 and uPA might be involved in the activation of pro-MMP-13 through unknown mechanisms in arthritic diseases.




Similar content being viewed by others
References
Mor A, Abramson SB, Pillinger MH (2005) The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol 115(2):118–128
Weinberg JB, Wortham TS, Misukonis MA, Patton KL, Chitneni SR (1993) Synovial mononuclear phagocytes in rheumatoid arthritis and osteoarthritis: quantitative and functional aspects. Immunol Invest 22(5):365–374
Feldmann M, Brennan FM, Maini RN (1996) Rheumatoid arthritis. Cell 85(3):307–310
Murphy G, Knauper V, Atkinson S, Butler G, English W, Hutton M et al (2002) Matrix metalloproteinases in arthritic disease. Arthritis Res 4(Suppl 3):S39–S49
Burrage PS, Mix KS, Brinckerhoff CE (2006) Matrix metalloproteinases: role in arthritis. Front Biosci 11:529–543
Vincenti MP, Brinckerhoff CE (2002) Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res 4(3):157–164
Knauper V, Cowell S, Smith B, Lopez-Otin C, O’Shea M, Morris H et al (1997) The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem 272(12):7608–7616
Krane SM, Byrne MH, Lemaitre V, Henriet P, Jeffrey JJ, Witter JP et al (1996) Different collagenase gene products have different roles in degradation of type I collagen. J biol chem 271(45):28509–28515
Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G (1996) Biochemical characterization of human collagenase-3. J Biol Chem 271(3):1544–1550
Hu B, Kapila YL, Buddhikot M, Shiga M, Kapila S (2000) Coordinate induction of collagenase-1, stromelysin-1 and urokinase plasminogen activator (uPA) by the 120-kDa cell-binding fibronectin fragment in fibrocartilaginous cells: uPA contributes to activation of procollagenase-1. Matrix Biol 19(7):657–669
Nagase H, Suzuki K, Morodomi T, Enghild JJ, Salvesen G (1992) Activation mechanisms of the precursors of matrix metalloproteinases 1, 2 and 3. Matrix Suppl 1:237–244
Murphy G, Knauper V (1997) Relating matrix metalloproteinase structure to function: why the “hemopexin” domain? Matrix Biol 15(8–9):511–518
Muroski ME, Roycik MD, Newcomer RG, Van den Steen PE, Opdenakker G, Monroe HR et al (2008) Matrix metalloproteinase-9/gelatinase B is a putative therapeutic target of chronic obstructive pulmonary disease and multiple sclerosis. Curr Pharm Biotechnol 9(1):34–46
Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M (1996) Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett 393(1):101–104
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Weiss SJ, Peppin G, Ortiz X, Ragsdale C, Test ST (1985) Oxidative autoactivation of latent collagenase by human neutrophils. Science 227(4688):747–749
Fu X, Kassim SY, Parks WC, Heinecke JW (2003) Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J Biol Chem 278(31):28403–28409
Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ (1996) Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum 39(9):1576–1587
Gruber BL, Sorbi D, French DL, Marchese MJ, Nuovo GJ, Kew RR et al (1996) Markedly elevated serum MMP-9 (gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker. Clin Immunol Immunopathol 78(2):161–171
Prince HE (2005) Biomarkers for diagnosing and monitoring autoimmune diseases. Biomarkers 10(Suppl 1):S44–S49
Miller MC, Manning HB, Jain A, Troeberg L, Dudhia J, Essex D et al (2009) Membrane type 1 matrix metalloproteinase is a crucial promoter of synovial invasion in human rheumatoid arthritis. Arthritis Rheum 60(3):686–697
Hayashi N, Nishimura K, Kumagai S (2008) New biomarkers for rheumatoid arthritis. Rinsho Byori 56(4):297–308
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K et al (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737–744
Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R (2002) The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol 169(5):2643–2647
Kim KS, Choi HM, Lee YA, Choi IA, Lee SH, Hong SJ et al (2011) Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol Int 2011 31(4):543–547
Santos LL, Morand EF, Hutchinson P, Boyce NW, Holdsworth SR (1997) Anti-neutrophil monoclonal antibody therapy inhibits the development of adjuvant arthritis. Clin Exp Immunol 107(2):248–253
Busso N, Peclat V, So A, Sappino AP (1997) Plasminogen activation in synovial tissues: differences between normal, osteoarthritis, and rheumatoid arthritis joints. Ann Rheum Dis 56(9):550–557
Li J, Ny A, Leonardsson G, Nandakumar KS, Holmdahl R, Ny T (2005) The plasminogen activator/plasmin system is essential for development of the joint inflammatory phase of collagen type II-induced arthritis. Am J Pathol 166(3):783–792
Jin T, Tarkowski A, Carmeliet P, Bokarewa M (2003) Urokinase, a constitutive component of the inflamed synovial fluid, induces arthritis. Arthritis Res Ther 5(1):R9–R17
Drummond AH, Beckett P, Brown PD, Bone EA, Davidson AH, Galloway WA et al (1999) Preclinical and clinical studies of MMP inhibitors in cancer. Ann N Y Acad Sci 878:228–235
Purcell WT, Rudek MA, Hidalgo M (2002) Development of matrix metalloproteinase inhibitors in cancer therapy. Hematol Oncol Clin North Am 16(5):1189–1227
Brown PD (1999) Clinical studies with matrix metalloproteinase inhibitors. APMIS 107(1):174–180
Rossello A, Nuti E, Catalani MP, Carelli P, Orlandini E, Rapposelli S et al (2005) A new development of matrix metalloproteinase inhibitors: twin hydroxamic acids as potent inhibitors of MMPs. Bioorg Med Chem Lett 15(9):2311–2314
Devel L, Rogakos V, David A, Makaritis A, Beau F, Cuniasse P et al (2006) Development of selective inhibitors and substrate of matrix metalloproteinase-12. J Biol Chem 281(16):11152–11160
Acknowledgments
This study was supported by a grant from the Kyung Hee University in 2010 (KHU-20110062).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, K.S., Lee, YA., Choi, H.M. et al. Implication of MMP-9 and urokinase plasminogen activator (uPA) in the activation of pro-matrix metalloproteinase (MMP)-13. Rheumatol Int 32, 3069–3075 (2012). https://doi.org/10.1007/s00296-011-2095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-011-2095-4