Abstract
In this work, Ag0.5Cr2.5O4 nanochromite is fabricated utilizing a simple process (flash technique) at various annealing temperatures (room and 900 °C). The particle sizes of the materials under study were shown to be in the nanoscale range by atomic force microscopy (AFM) and field emission scanning electron microscopy (FESEM). Fourier transform infrared (FTIR) analysis was performed to verify the fabrication of the examined nanosamples and evaluate the bands behavior. The tetrahedral A-site (622.9 cm−1 for room temperature, 630.6 cm−1 for 900 °C) and the octahedral B-site (557.3 cm−1 for room temperature, 563.1 cm−1 for 900 °C) were the two prominent bands measured by FTIR analysis. The elastic characteristics of Ag0.5Cr2.5O4 nanoparticles were examined using FTIR measurements, revealing that the interatomic bonding of the atoms at 900 °C is higher than at room temperature. In addition, the elastic characteristics may be understood by analyzing the transverse and longitudinal velocities. Both Gram-positive and Gram-negative bacteria were effectively inhibited by the samples evaluated for antibacterial properties; however, neither sample showed any antifungal activity. Therefore, it is highly suggested that the investigated samples could be used in different applications, particularly biological ones.
Similar content being viewed by others
Introduction
Silver is an example of a noble metal used in several industries, including electrical, water treatment, antiviral/antibacterial research, and solar cell manufacturing [1,2,3,4,5]. Nanochromites are very stable at high annealing temperatures, making them suitable for various technological uses [6]. The study of the physical characteristics of nanoparticles is vital for elucidating the sample’s behavior and determining the most appropriate use for the sample [7,8,9,10]. Selecting the best technique for improving products is essential. In this research, nanoparticles were created using a flash auto-combustion technique for excellent stoichiometric regulation. In addition, it is a simple, low-cost, and quick procedure that saves time [6, 11]. The recent irresponsible overuse of antibiotics has increased the urgency of finding effective alternatives, leading to the rise in the popularity of silver complexes and other antimicrobial applications [12,13,14,15]. Therefore, an antibacterial study used both Gram-positive and Gram-negative bacteria in addition to fungi for testing.
X-ray examination of Ag0.5Cr2.5O4 nanoparticles at room temperature and 900 °C revealed a cubic structure, as described in our previous work [16, 17]. The nature of the binding forces in solids may be elucidated by measuring elastic constants such as Poisson’s ratio, Young’s modulus, rigidity, and bulk modulus. FTIR analysis may be used to learn about elastic properties, and it can reveal the Debye temperature and the longitudinal velocity, among other elastic constants. Moreover, the X-ray diffraction pattern parameters, combined with those of FTIR spectroscopy, are significant when calculating certain elastic moduli, such as shear and sound velocities [18, 19]. This enables the measurement of elastic characteristics to estimate the interatomic bonding strength. According to the authors’ knowledge, no previous study has been conducted on the elastic properties of Ag0.5Cr2.5O4 nanochromite. It is reported from our previous work that silver chromite with the chemical formula Ag2Cr2O4 nanoparticles with the ratio of Ag/Cr ion (1:1) at room temperature is stronger than Ag2Fe2O4 nanoparticles with the ratio of Ag/Fe ion (1:1) [20]. Also, when comparing silver chromite with different chemical formulas AgCrO2 with the ratio of Ag/Cr ion (1:1) at room temperature gave high strength than AgFeO2 with the ratio of Ag/Fe ion (1:1) [21]. This indicates that chromium ions possess more elasticity than iron ions, which could be used in various applications. The author chose to add chromium to silver over iron because chromium’s elasticity is higher than iron’s. The authors decided to increase the ratio of chromium to silver by 5:1 to ensure that when the element of chromium is increased over iron, the elasticity will increase, and this has already been confirmed in this research. The authors also studied the mechanical properties of the investigated material with increasing temperature. Thus, the authors study the mechanical properties of silver chromite by increasing the chromite concentration (Cr2.5) at the expense of decreasing silver ion concentration (Ag0.5) with the ratio of Ag/Cr ion (1:5) compared with previous work (Ag2Cr2O4 and AgCrO2) with the ratio of Ag/Cr (1:1) [20, 21]. Moreover, the authors studied the effect of the investigated samples with various temperatures, and they found that by increasing the annealing temperature, the elasticity of the nanosample increased.
Consequently, this research aims to explore the FTIR analysis of Ag0.5Cr2.5O4 nanochromite, the elastic properties derived from infrared analysis at various annealing temperatures, and the antibacterial application of the investigated materials. The study of the elastic properties of the investigated samples is novel, and this is the first time to illustrate the mechanical properties of Ag0.5Cr2.5O4 nanoparticles at different annealing temperatures from FTIR analysis. An illustration of elastic properties can give a complete description of the samples that could be appropriately used in many technological applications.
Experimental approaches
Preparation and analysis
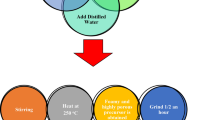
Nanochromite (Ag0.5Cr2.5O4) was synthesized by a simple technique (flash technique). Nitrate salts, such as chromium nitrate (100.04 g Cr (NO3)3) and silver nitrate (8.49 g AgNO3), are the starting materials. Urea (20 g) and some distilled water were added to the mixture, as shown in Fig. 1. The mixture is then heated to 250 °C to become a powder. The powder used in the analyses was annealed for 2 hours at two different temperatures (room temperature and 900 °C) as the last stage in this experiment. The FTIR (Fourier transform infrared) analysis was performed between 400 and 4000 cm−1 using a spectrometer manufactured in Japan called a Jasco FTIR 300 E. The Wet-PM-9600, a Japanese-made atomic force microscopy instrument, was used at room temperature to take the measurements in a non-contact mode. The field emission scanning electron microscopy (FESM) was measured using a 250 FEG quanta model.
Antimicrobial measurement
The Kirby-Bauer technique [22] with a modified disk was used for the in vitro examination of the antibacterial activity. Staphylococcus aureus ATCC 12,600, Bacillus subtilis ATCC 6051, and Streptococcus faecalis ATCC 19,433 were Gram-positive bacteria strains examined and analyzed. The Gram-negative bacteria tested were Pseudomonas aeruginosa ATCC 10,145, Escherichia coli ATCC 11,775, and Neisseria gonorrhea ATCC 19,424. Candida albicans ATCC 7102 and Aspergillus flavus link were also tested for their fungal properties. The nanosample Ag0.5Cr2.5O4 was used against the tested bacteria and fungi. A 100 µl of tested bacteria or fungi were spread on the agar surface to form a colony, and then the investigated sample was added to it. The Petri plates were cultured at 30° C for 24 and 48 h. The inhibition zone diameters were measured to assess the efficacy of the substances tested against the bacteria and fungi utilized in the experiment. Ampicillin and Amphotericin B were considered as antibacterial and antifungal control to be compared with them.
Results and discussion
AFM study
Figure 2a–c shows the atomic force microscopy (AFM) study of the surface morphology of Ag0.5Cr2.5O4 nanoparticles at 900 °C. The figures show that agglomeration occurred in the tested sample due to no surfactant used during the preparation of the nanoparticles [23, 24], as shown in Fig. 2a. Table 1 shows the values of the particle size and roughness. The AFM micrograph was analyzed using the image editing tool ImageJ to generate the average particle size histogram and a roughness histogram, as shown in Fig. 2b, c, respectively. Finally, the average particle sizes of the examined samples were found to be on the nanoscale.
FESEM and EDX study
Figure 3a, b shows the field emission scanning electron microscopy (FESEM) study of the investigated samples at various annealing temperatures. Table 1 shows that the particle size of both samples estimated from the FESEM image was in the nanoscale range. The agglomeration appears in Fig. 3a, b, especially at Ag0.5Cr2.5O4 nanoparticles at 900 °C, since the surfactant was not added during the synthesis of the samples [25]. Thus, the FESEM test assured the nanoscale range of the investigated samples.
Figure 3c and Table 2 show energy-dispersive X-ray analysis (EDX) of Ag0.5Cr2.5O4 nanoparticles at room temperature. The values showed the homogeneity mixing of Ag, O, and Cr atoms. Moreover, there is a good agreement between theoretical and experimental composition values of weight percentage (wt%) and atomic percentage (at.%) of the investigated sample.
FTIR Study
In general, in solids, ion vibrations in the crystal lattice are attributed to infrared bands [26]. Fourier transform infrared spectroscopy (FTIR) is utilized to examine the structural modifications of Ag0.5Cr2.5O4 nanoparticles at room temperature and 900 °C in the wave number range of 400–4000 cm−1, as shown in Fig. 4 and Table 3. One of the primary strong absorption bands in the FTIR spectra of both materials is at approximately 500 cm−1 (peak 1), while the other is at around 600 cm−1 (peak 2). The shorter distance between Cr3+ and O2− atoms in octahedral complexes, relative to tetrahedral complexes [27,28,29,30], may account for the observed band position differences. As the annealing temperature is raised, the crystallinity is improved. The tetrahedral absorption peak of the Ag0.5Cr2.5O4 nanoparticle at 900 °C shifts slightly toward the higher frequency side relative to that at room temperature. The C–O–C asymmetric stretching vibration causes the spectral peak at location (3). The band at peak (4) suggests that C=C stretching vibration occurs. The stretching vibration of the C=O and carboxyl groups may also be responsible for the band at peak 5. The band at peak (6) is assumed to be generated by the absorption of atmospheric CO2 on the surface of the nanoparticles during the FTIR test. The band at peak (7) may be related to the stretching vibration of the C–H group. The strong band at peak (8) may be attributed to water symmetric stretching vibration and NH group.
Elastic Study
Elastic constants are essential in measuring the resistance of a crystal to an externally applied stress. Thus, the elastic properties of nanoparticles play an essential role in industrial applications [26, 31]. FTIR analysis was used to examine the elastic properties of Ag0.5Cr2.5O4 nanoparticles at room temperature and 900 °C, as reported by Modi et al. [31]. The tetrahedral and octahedral force constants, Kt and Ko, were calculated by the following equations [28]:
where “MA” denotes the tetrahedral A-site molecular weight and “MB” is the octahedral B-site molecular weight. The reported values for the force constants Kt and Ko are shown in Table 4. It has been shown that Kt values are higher than Ko values. Table 4 shows that the values of the force constants Kt decrease and Ko increase as the crystallite size increases owing to fluctuations in cation-oxygen bond lengths at the A- and B-site [28].
To determine the conduction mechanism of the tested samples, the Debye temperature D should be calculated as follows [32]:
where c represents the speed of light (3 × 1010 cm/s), kB represents the Boltzmann constant, vav represents the average frequency, h represents the Plank constant, and νt and νo represent the frequencies of the tetrahedral A-site and the octahedral B-site, respectively. As can be observed, the values of the Debye temperature play a significant role in determining the conduction mechanism of these nanoparticles.
It can be shown that the slightly higher θD values occurred from increasing the annealing temperature of the investigated samples. From Anderson’s formula [33], we obtained the following formula for calculating the Debye temperature:
where VA stands for the mean atomic volume, M for the molecular weight, q for the number of atoms in one unit of the formula (q = 7), and NA for the Avogadro number. It was found that increasing the annealing temperature of the nanosamples caused an increase in \(\theta_{{\text{D}}}^{\prime }\). This increase is described according to the increase of mean wave velocity Vm. The equation to compute the bulk modulus B of solids [34] is:
where C11 and C12 are the constants used to measure stiffness. From the following relation [35], it can be shown that there is a linkage between the average force constant K and the stiffness constant C11:
Also, the values of the longitudinal elastic wave velocity, also known as Vl, the transverse or shear elastic wave velocity, also known as Vs = Vt, and the mean elastic wave velocity, known as Vm, are provided by the following relations [36]:
In addition to this, the values of the longitudinal modulus (L), Young’s modulus (E), rigidity modulus (G), and Poisson’s ratio (σ) may be determined using the following relations [37]:
All the values of the elastic parameters that were measured on the samples under investigation are shown in Tables 4, 5. Raising the annealing temperature of the investigated samples shows that the values for L, G, B, and E increased. The concept of interatomic bonding may be used to explain this phenomenon. This indicates that the interatomic bonding of the atoms at 900 °C is much stronger than that at room temperature. In addition to this, the values of Poisson’s ratio (σ) remain constant by increasing the annealing temperature of the investigated samples (σ = 0.25), and they are in good agreement with the theory of isotropic elasticity, which had a range that went from − 1 to 0.5 [38]. Finally, studying the mechanical properties of Ag0.5Cr2.5O4 nanoparticles with the ratio of Ag/Cr (1:5) gave more elastic behavior than that of previous work with a ratio of Ag/Cr (1:1) [21]. Moreover, by increasing the annealing temperature of the investigated samples to 900 °C, an enhancement happened in the mechanical properties, especially in the elasticity of the nanosample.
Antimicrobial study
Figure 5a, b illustrates the nanoparticles of the investigated sample Ag0.5Cr2.5O4 nanoparticles at room temperature in vitro. These figures depict Gram-positive, Gram-negative, and fungal microorganisms and the diameters of their inhibition zones, as described in Table 6. This table demonstrates that Ag0.5Cr2.5O4 nanoparticles had a good antibacterial impact against Gram-positive microbes and Gram-negative microorganisms compared to the antibacterial antibiotic ampicillin. It is observed from Table 6 that the tested bacteria Staphylococcus aureus, Streptococcus faecalis, and Pseudomonas aeruginosa show higher efficacy than the other tested bacteria. When the studied material was added to the tested microorganisms, the cell membrane of the bacteria was disrupted [39,40,41,42,43,44,45,46,47] due to the presence of toxic silver and chromium ions. Adding Ag0.5Cr2.5O4 nanoparticles, on the other hand, had no impact on fungal germs. Our earlier research [17] showed the antibacterial properties of Ag0.5Cr2.5O4 nanoparticles at 900 °C in detail. Compared to Ag0.5Cr2.5O4 nanoparticles at 900 °C from our previous study [17] and Ag0.5Cr2.5O4 nanoparticles at room temperature from the current work, Ag0.5Cr2.5O4 nanoparticles at 900 °C were stronger against Gram-positive and Gram-negative bacteria than that at room temperature. This may be due to the smaller crystallite size at 900 °C than at room temperature. On the other hand, both samples did not affect the tested fungi. Thus, the investigated samples are highly recommended as an antibacterial agent, particularly against the tested Gram-positive and Gram-negative bacteria.
Conclusion
-
1.
We effectively prepared Ag0.5Cr2.5O4 nanoparticles at various annealing temperatures using a simple and inexpensive technique.
-
2.
The morphology reveals that the crystallite size and particle size of Ag0.5Cr2.5O4 nanoparticles are in the nanoscale range.
-
3.
From the elastic study, Ag0.5Cr2.5O4 nanoparticles at 900 °C showed a higher elasticity than at room temperature.
-
4.
Both samples showed effective behavior against tested Gram-positive and Gram-negative bacteria.
-
5.
Ag0.5Cr2.5O4 nanoparticles at room temperature and 900 °C are innovative materials that have the potential to be incorporated into a variety of medications.
-
6.
In conclusion, the sample Ag0.5Cr2.5O4 nanoparticles at different annealing temperatures are strongly recommended to be applied in biomedical applications.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Honary S, Ghajar K, Khazaeli P, Schalchian P (2011) Preparation, characterization and antibacterial properties of silver-chitosan nanocomposites using different molecular weight grades of chitosan. Trop J Pharm Res 10:69–74
Gamal WM, El-Bassuony AAH, Hafez RS, Abdelsalam HK (2022) Study of the structural and magnetic properties of a novel cola/lah nanocomposite material. JOM 74:4898–4908. https://doi.org/10.1007/s11837-022-05491-x
Abdelhamid HN, Talib A, Wu H-F (2015) Facile synthesis of water-soluble silver ferrite (AgFeO2) nanoparticles and their biological application as antibacterial agents. RSC Adv 5:34594–34602
El-Bassuony AAH, Abdelsalam HK (2019) Tailoring the structural, magnetic and antimicrobial activity of AgCrO2 delafossite at high annealing temperature. J Therm Anal Calorim 138:81–88. https://doi.org/10.1007/s10973-019-08207-7
Anooj ES, Sreelekshmi SJ, Gopukumar ST, Praseetha PK (2017) Evaluation of the zinc ferrite nano particles for bio-applications. Int J Pharm Sci Rev Res 46:22
Sayed MA, El-Rahman TMAA et al (2022) Attractive study of the antimicrobial, antiviral, and cytotoxic activity of novel synthesized silver chromite nanocomposites. BMC Chem 16:39. https://doi.org/10.1186/s13065-022-00832-y
Nangmenyi G, Li X, Mehrabi S, Mintz E, Economy J (2011) Silver-modified iron oxide nanoparticle impregnated fiberglass for disinfection of bacteria and viruses in water. Mater Lett 65:1191
Kaskhedikar NA, Maier J (2009) Lithium storage in carbon nanostructures. Adv Mater 21:2664
Naseri MG, Saion EB, Ahangar HA, Shaari AH (2013) Fabrication, characterization, and magnetic properties of copper ferrite nanoparticles prepared by a simple, thermal-treatment method. Mater Res Bull 48:1439
Li H, Chen Q, Zhao J, Urmila K (2015) Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci Rep 5:11033
Sayed MA, El-Bassuony AAH, Abdelsalam HK (2020) Evaluation of antimicrobial properties of a novel synthesized nanometric delafossite. Braz J Microbiol 51:1475–1482. https://doi.org/10.1007/s42770-020-00366-2
Nomiya K, Yoshizawa A, Tsukagoshi K, Kasuga NC, Hirakawa S, Watanabe J (2004) Synthesis and structural characterization of silver(I), aluminium(III) and cobalt(II) complexes with 4-isopropyltropolone (hinokitiol) showing noteworthy biological activities. Action of silver (I)-oxygen bonding complexes on the antimicrobial activities. J Inorg Biochem 98:46–60
El-Bassuony AAH (2020) Effect of Al addition on structural, magnetic, and antimicrobial properties of Ag nanoparticles for biomedical applications. JOM 72:1154–1162. https://doi.org/10.1007/s11837-019-03784-2
Huang Z, Jiang X, Guo D, Gu N (2011) Controllable synthesis and biomedical applications of silver nanomaterials. J Nanosci Nanotechnol 11:9395–9408
Paulsen JA, Lo CCH, Snyder JE, Ring AP, Jones LL, Jiles DC (2003) Study of the Curie temperature of cobalt ferrite based composites for stress sensor applications. IEEE Trans Magn 39:3316
El-Bassuony AAH, Abdelsalam HK (2018) Giant exchange bias of hysteresis loops on Cr3+-doped Ag nanoparticles. J Supercond Novel Magn 31:1539–1544. https://doi.org/10.1007/s10948-017-4340-x
El-Bassuony AAH (2020) Influence of high annealing temperature on structural, magnetic and antimicrobial activity of silver chromite nanoparticles for biomedical applications. J Inorg Organomet Polym Mater 30:1821–1828. https://doi.org/10.1007/s10904-019-01306-w
El-Ghazzawy EH (2020) Effect of heat treatment on structural, magnetic, elastic and optical properties of the co-precipitated Co0.4Sr0.6Fe2O4. J Magn Magn Mater 497:166017
Rao KS, Kumar AM, Varma MC, Choudary GSVRK, Rao KH (2009) Cation distribution of titanium substituted cobalt ferrites. J Alloys Compd 488:L6
Gamal WM, El-Bassuony AH, Abdelsalam HK et al (2021) Role of elastic and optical properties on silver nanoferrite and nanochromite for optical switch device applications. J Mater Sci Mater Electron 32:21590–21602. https://doi.org/10.1007/s10854-021-06667-y
El-Bassuony AAH, Abdelsalam HK, Gamal WM (2022) Influence of elastic and optical properties on AgFeO2 and AgCrO2 delafossite to be applied in high-frequency applications. JOM 74:2656–2664. https://doi.org/10.1007/s11837-022-05170-x
Bauer AW, Kirby WM, Sherris C, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Ajroudi L, Mliki N, Bessais L, Madigou V, Villain S, Leroux Ch (2014) Magnetic, electric and thermal properties of cobalt ferrite nanoparticles. Mater Res Bull 59:49
Ateia E, El-Bassuony AAH (2017) Fascinating improvement in physical properties of Cd/Co nanoferrites using different rare earth ions. J Mater Sci Mater Electron 28:11482–11490. https://doi.org/10.1007/s10854-017-6944-0
El-Bassuony AAH, Abdelsalam HK (2020) Correlation of heat treatment and the impurities accompanying Ag nanoparticles. Euro Phys J Plus 135:66. https://doi.org/10.1140/epjp/s13360-019-00025-y
Bouokkeze D, Massoudi J, Hzez W, Smari M, Bougoffa A, Khirouni K, Dhahri E, Bessais L (2019) Investigation of the structural, optical, elastic and electrical properties of spinel LiZn2Fe3O8 nanoparticles annealed at two distinct temperatures. RSC Adv 9:40940
Albalah MA, Alsabah YA, Mustafa DE (2020) Characteristics of co-precipitation synthesized cobalt nanoferrites and their potential in industrial wastewater treatment. SN Appl Sci 2:804
Waldron RD (1955) Infrared spectra of ferrites. Phys Rev 99:1727–1735
Mazen SA, Mansour SF, Dhahri E, Zaki HM, Elmosalami TA (2009) The infrared absorption and dielectric properties of Li-Ga ferrite. J Alloy Compd 470:294–300
Watawe SC, Sutar BD, Sarwade BD, Chougule BK (2001) Infrared studies of some mixed Li–Co ferrites. Int J Inorg Mater 3:819–823
Kuo MF, Hung YH, Huang JY, Huang CC (2016) Structure and magnetic properties of Mn and Al doped magnesium ferrite. China Steel Tech Rep 29:44
Modi KB, Trivedi UN, Pandya MP, Bhatu SS, Chhantbar MC, Joshi HH (2004) Microwaves and optoelectronics. Anamaya Publishers, New Delhi
Anderson OL (1965) Physical acoustics, vol III. Academic Press, New York
Modi KB, Trivedi UN, Pandya MP, Bhatu SS, Chhantbar MC, Joshi HH (2004) Study of elastic properties of magnesium and aluminum co-substituted lithium ferrite near microwave frequencies, in microwaves and optoelectronics. Anamaya Publishers, New Delhi, p 223
Ravinder D, Balachander L, Venudhar YC (2001) Elastic behaviour of manganese substituted lithium ferrites. Mater Lett 49:205
Patil VG, Shirsath SE, More SD et al (2009) Effect of zinc substitution on structural and elastic properties of cobalt ferrite. J Alloy Compd 488:199–203
Raj B, Rajendram V, Palanichamy P (2004) Science and technology of ultrasonics. Narosa Publishing House, New Delhi
Modi KB, Rangolia MK, Chhantbar MC, Joshi HH (2006) Study of infrared spectroscopy and elastic properties of fine and coarse grained nickel-cadmium ferrites. J Mater Sci 41:7308–7318
El-Bassuony AAH, Gamal WM, Abdelsalam HK (2022) Fascinating study of adding nanocomposite cobalt nano ferrite to silver nanoparticles accompanied magnetite impurity. J Mater Sci Mater Electron 33:16219–16235. https://doi.org/10.1007/s10854-022-08516-y
Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R (2015) Alternative antimicrobial approach: nano-antimicrobial materials. Evid Based Complement Altern Med 2015:246012. https://doi.org/10.1155/2015/246012
Akhtar MN, Saleem M, Khan MA (2018) Al doped spinel and garnet nanostructured ferrites for microwave frequency C and X-band applications. J Phys Chem Solids 123:260
El-Bassuony AAH, Abdelsalam HK (2023) Attractive study of the physical properties of silver iron oxide nanoparticles for biomedical applications. Phys. Scr. 98:055919. https://doi.org/10.1088/1402-4896/acc90c
Ali EAM, Sayed MA, Abdel-Rahman TMA, Hussein AM, Hussein R (2019) Bioremediation of waste water from cadmium pollution using silicon dioxide nanoparticles and fungal biomasses. J Pure Appl Microbiol 13:1561–1570. https://doi.org/10.22207/JPAM.13.3.29
El-Bassuony AAH, Gamal WM, Abdelsalam HK (2022) Influence of silver nanoferrite and nanochromite on physical properties for high-frequency and biomedical applications. JOM 74:2635–2644. https://doi.org/10.1007/s11837-022-05315-y
Baiomy AA (2016) Histopathological biomarkers and genotoxicity in gill and liver tissues of Nile tilapia Oreochromis niloticus from a polluted part of the Nile River. Egypt Afr J Aquat Sci 41:181–19127. https://doi.org/10.2989/16085914.2016.1168734
El-Bassuony AAH, Gamal WM, Abdelsalam HK (2023) Impact of different magnetic materials added to silver-magnetite nanoparticles on the structural, magnetic and antimicrobial properties. Euro Phys J Spec Top. https://doi.org/10.1140/epjs/s11734-022-00759-4
Alkhedaide AQH, Alshehri ZS, Soliman MM, Althumali KW, Abu-Elzahab HS, Baiomy AA (2016) Vitamin D3 supplementation improves immune and inflammatory response in vitamin D deficient adults in Taif, Saudi Arabia. Biomed Res (India) 27:1049–1053
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting, and revising of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gamal, W.M., El-Bassuony, A.A.H. & Abdelsalam, H.K. Fascinating study of elastic, FTIR, and antimicrobial properties of silver nanochromite at different annealing temperatures. Polym. Bull. 81, 1821–1837 (2024). https://doi.org/10.1007/s00289-023-04788-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04788-4