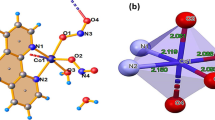
Abstract
Long-term excessive uptake of fluoride through potable water will cause adverse effects on human health. Therefore, it is of great significance to detect and remove excessive fluoride in potable water. Here, we reported a multi-functional Zr-based coordination polymer namely CP-1, which could realize the visual detection and effectively selective adsorption of fluoride. The investigation of fluoride adsorption manifested that the maximum fluoride adsorption capacity of CP-1 could reach 48.78 mg g−1 and selective adsorption capacity kept stable in a wide range of pH. By fluorometric approaches, the detection of limit of F− could achieve 1.55 × 10–6 mol L−1 based on CP-1. Moreover, the fluorescence test paper fabricated by in situ growth of CP-1 onto the surface of filter paper was proved to realize the visual detection of fluoride.
Graphical abstract








Similar content being viewed by others
References
Tan TL, Krusnamurthy PP, Nakajima H, Rashid SA (2020) Adsorptive, kinetics and regeneration studies of fluoride removal from water using zirconium-based metal organic frameworks. RSC Adv 10(32):18740–18752
Jain A (2014) Singh S (2014) Prevalence of Fluoride in Ground Water in Rajasthan State: Extent, Contamination Levels and Mitigation. Open J Water Pollut Treat 2:50–57
Fath A, Sacher F, McCaskie JE (2016) Electrochemical decomposition of fluorinated wetting agents in plating industry waste water. Water Sci Technol 73(7):1659–1666
Huang L, Wan K, Yan J, Wang L, Li Q, Chen H, Zhang H, Xiao T (2021) Nanomaterials in water applications: adsorbing materials for fluoride removal. Nanomaterials 11:1866. https://doi.org/10.3390/nano11071866
Rawlani S, Rawlani S, Rawlani S (2010) Assessment of skeletal and non-skeletal fluorosis in endemic fluoridated areas of vidharbha region, india: a survey. Ind J Commun Med 35(2):298–301
Ghosh A, Mukherjee K, Ghosh SK, Saha B (2012) Sources and toxicity of fluoride in the environment. Res Chem Intermed 39(7):2881–2915
Javanbakht S, Pooresmaeil M, Namazi H (2019) Green one-pot synthesis of carboxymethyl cellulose/Zn-based metal-organic framework/grapheme oxide bio-nanocomposite as a nano-carrier for drug delivery system. Carb Polym 208:294–301
Shen J, Mkongo G, Abbt-Braun G, Ceppi SL, Richards BS, Schäfer AI (2015) Renewable energy powered membrane technology: Fluoride removal in a rural community in northern Tanzania. Sep Purif Technol 149:349–361
Robshaw TJ, Dawson R, Bonser K, Ogden MD (2019) Towards the implementation of an ion-exchange system for recovery of fluoride commodity chemicals. Kinetic and dynamic studies. Chem Eng J 367:149–159
Wu X, Zhang Y, Dou X, Zhao B, Yang M (2013) Fluoride adsorption on an Fe-Al-Ce trimetal hydrous oxide: characterization of adsorption sites and adsorbed fluorine complex species. Chem Eng J 223:364–370
Gai WZ, Deng ZY (2021) A comprehensive review of adsorbents for fluoride removal from water: performance, water quality assessment and mechanism. Environ Sci: Water Res Technol 7(8):1362–1386
Ahmadijokani F, Molavi H, Rezakazemi M, Aminabhavi TM, Arjmand M (2021) Simultaneous detection and removal of fluoride from water using smart metal-organic framework-based adsorbents. Coord Chem Rev 445:214037
Pillai P, Dharaskar S, Pandian S, Panchal H (2021) Overview of fluoride removal from water using separation techniques. Environ Technol Innov 21:101246
Chidambaram S, Manikandan S, Ramanathan AL, Prasanna MV, Thivya C, Karmegam U, Thilagavathi R, Rajkumar K (2013) A study on the defluoridation in water by using natural soil. Appl Water Sci 3(4):741–751
Cheng J, Meng X, Jing C, Hao J (2014) La3+-modified activated alumina for fluoride removal from water. J Hazard Mater 278:343–349
Prasad KSB, Jakeer HP, Bharath KP (2021) Defluorination of groundwater by low-cost adsorbents. IOP Conf Series: Mater Sci Eng. 1025(1):012033
Zhu L, Zhang C, Wang L, Zhang J (2022) The simple synthesis of metal organic frameworks with high fluoride adsorption performance from water. J Solid State Chem 307:122866
Fonseca J, Gong T, Jiao L, Jiang HL (2021) Metal-organic frameworks (MOFs) beyond crystallinity: amorphous MOFs, MOF liquids and MOF glasses. J Mater Chem A 9(17):10562–10611
Abdelhamid HN, Mathew AP (2022) Cellulose-metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted Applications: A review. Coord Chem Rev 451:214263
Shen K, Chen X, Chen J, Li Y (2016) Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal 6(9):5887–5903
Zhang Y, Feng X, Yuan S, Zhou J, Wang B (2016) Challenges and recent advances in MOF-polymer composite membranes for gas separation. Inorganic Chemistry Frontiers 3(7):896–909
Torad NL, Li Y, Ishihara S, Ariga K, Kamachi Y, Lian HY, Hamoudi H, Sakka Y, Chaikittisilp W, Wu KCW, Yamauchi Y (2014) MOF-derived Nanoporous Carbon as Intracellular Drug Delivery Carriers. Chem Lett 43(5):717–719
Yassine O, Shekhah O, Assen AH, Belmabkhout Y, Salama KN, Eddaoudi M (2016) H2S Sensors: Fumarate-Based fcu-MOF Thin Film Grown on a Capacitive Interdigitated Electrode. Angew Chem Int Ed Engl 55(51):15879–15883
Ghanbari T, Abnisa F, Wan D, W. M A, (2020) A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci Total Environ 707:135090
Liu X, Pang H, Liu X, Li Q, Zhang N, Mao L, Qiu M, Hu B, Yang H, Wang X (2021) Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. Innovation (N Y) 2(1):100076
Haldar D, Duarah P, Purkait MK (2020) MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 251:126388
Antonysamy J, Ilango K, Mu N, Natrayasamy V (2022) Fabrication of hydroxyapatite embedded cerium-organic frameworks for fluoride capture from water. J Mol Liq 354:118830
Wang X, Zhu H, Sun T, Liu Y, Han T, Lu J, Dai H, Zhai L(2019) Synthesis and study of an efficient metal-organic framework adsorbent (MIL-96(Al)) for fluoride removal from water. J Nanomater 3128179.
Ahmadijokani F, Molavi H, Rezakazemi M, Tajahmadi S, Bahi A, Frank K, Aminabhavi TM, Li JR (2022) UiO-66 metal-organic frameworks in water treatment: a critical review. Prog Mater Sci 125:100904
Kumar S, Jain S, Nehra M, Dilbaghi N, Marrazza G, Kim KH (2020) Green synthesis of metal-organic frameworks: a state-of-the-art review of potential environmental and medical applications. Coord Chem Rev 420:213407
Ahmad K, Nazir MA, Qureshi AK, Hussain E, Najam T, Javed MS, Shah SSA, Tufail MK, Hussain S, Khan NA, Shah HR, Ashfaq M (2020) Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater Sci Eng, B 262:114766
Bon V, Senkovska I, Evans JD, Wöllner M, Hölzel M, Kaskel S (2019) Insights into the water adsorption mechanism in the chemically stable zirconium-based MOF DUT-67- a prospective material for adsorption-driven heat transformations. Journal of Materials Chemistry A 7(20):12681–12690
Drache F, Bon V, Senkovska I, Marschelke C, Synytska A, Kaskel S (2016) Postsynthetic inner-surface functionalization of the highly stable zirconium-based metal-organic framework DUT-67. Inorg Chem 55(15):7206–7213
Kandiah M, Nilsen MH, Usseglio S, Jakobsen S, Olsbye U, Tilset M, Larabi C, Bonino Quadrelli EA (2010) Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem Mater 22(24):6632–6640
Zhu XH, Yang CX, Yan XP (2018) Metal-organic framework-801 for efficient removal of fluoride from water. Microporous Mesoporous Mater 259:163–170
Haddad PR, Nesterenko PN, Buchberger W (2008) Recent developments and emerging directions in ion chromatography. J Chromatogr A 1184(1–2):456–473
Rocha RA, Rojas D, Clemente MJ, Ruiz A, Devesa V, Velez D (2013) Quantification of fluoride in food by microwave acid digestion and fluoride ion-selective electrode. J Agric Food Chem 61(45):10708–10713
Zhu CQ, Chen JL, Zheng H, Wu YQ, Xu JG (2005) A colorimetric method for fluoride determination in aqueous samples based on the hydroxyl deprotection reaction of a cyanine dye. Anal Chim Acta 539(1–2):311–316
Zhu B, Fan C, Xu C, Wang L, Bi C, Zhang X, Fan Y (2021) Multi-responsive luminescent sensors of two water-stable polynuclear Cd organic frameworks: Synthesis, structures and sensing of tetracycline, Cr2O72− and Fe3+ ions in water. Microchem J 162:105880
Zhou Y, Huang X, Liu C, Zhang R, Gu X, Guan G, Jiang C, Zhang L, Du S, Liu B, Han MY, Zhang Z (2016) Color-multiplexing-based fluorescent test paper: dosage-sensitive visualization of Arsenic(III) with discernable scale as low as 5 ppb. Anal Chem 88(12):6105–6109
Gao M, Wang W, Yang H, Ye BC (2020) Efficient removal of fluoride from aqueous solutions using 3D flower-like hierarchical zinc-magnesium-aluminum ternary oxide microspheres. Chem Eng J 380:122459
Jeyaseelan A, Naushad M, Ahamad T, Viswanathan N (2021) Design and development of amine functionalized iron based metal organic frameworks for selective fluoride removal from water environment. J Environ Chem Eng 9(1):104563
Kuncoro EP, Isnadina DRM, Darmokoesoemo H, Fauziah OR, Kusuma HS (2018) Characterization, kinetic, and isotherm data for adsorption of Pb(2+) from aqueous solution by adsorbent from mixture of bagasse-bentonite. Data Brief 16:622–629
Kuncoro EP, Mitha Isnadina DR, Darmokoesoemo H, Dzembarahmatiny F, Kusuma HS (2018) Characterization and isotherm data for adsorption of Cd(2+) from aqueous solution by adsorbent from mixture of bagasse-bentonite. Data Brief 16:354–360
Neolaka YAB, Lawa Y, Naat J, Riwu AAP, Lindu YE, Darmokoesoemo H et al (2021) Evaluation of magnetic material IIP@GO-Fe3O4 based on Kesambi wood (Schleichera oleosa) as a potential adsorbent for the removal of Cr(VI) from aqueous solutions. React Funct Polym 166:105000
Neolaka YAB, Lawa Y, Naat J, Riwu AAP, Mango AW, Darmokoesoemo H et al (2022) Efficiency of activated natural zeolite-based magnetic composite (ANZ-Fe3O4) as a novel adsorbent for removal of Cr(VI) from wastewater. J Market Res 18:2896–2909
Neolaka YAB, Supriyanto G, Darmokoesoemo H, Kusuma HS (2018) Characterization, kinetic, and isotherm data for Cr(VI) removal from aqueous solution by Cr(VI)-imprinted poly(4-VP-co-MMA) supported on activated Indonesia (Ende-Flores) natural zeolite structure. Data Brief 17:969–979
Neolaka YAB, Supriyanto G, Darmokoesoemo H, Kusuma HS (2018) Characterization, isotherm, and thermodynamic data for selective adsorption of Cr(VI) from aqueous solution by Indonesia (Ende-Flores) natural zeolite Cr(VI)-imprinted-poly(4-VP-co-EGDMA)-ANZ (IIP-ANZ). Data Brief 17:1020–1029
Simonin JP (2016) On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem Eng J 300:254–263
Pan B, Xing B (2010) Adsorption kinetics of 17 α estradiol and bisphenol a on carbon nanomaterials I. several concerns regarding pseudo-first order and pseudo-second order models. J Soils Sediments. 10(5):838–844
Aigbe UO, Ukhurebor KE, Onyancha RB, Osibote OA, Darmokoesoemo H, Kusuma HS (2021) Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review. J Market Res 14:2751–2774
Neolaka YAB, Lawa Y, Naat J, Riwu AAP, Darmokoesoemo H, Widyaningrum BA et al (2021) Indonesian Kesambi wood (Schleichera oleosa) activated with pyrolysis and H2SO4 combination methods to produce mesoporous activated carbon for Pb(II) adsorption from aqueous solution. Environ Technol Innov 24:101997
Ghosal PS, Gupta AK (2017) Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J Mol Liq 225:137–146
Wang X, Cui S, Yan B, Wang L, Chen Y, Zhang J (2019) Isothermal adsorption characteristics and kinetics of Cr Ions onto Ettringite. J Wuhan Univer Technol Mater. Sci Ed. 34(3):587–595
Massoudinejad M, Ghaderpoori M, Shahsavani A, Amini MM (2016) Adsorption of fluoride over a metal organic framework Uio-66 functionalized with amine groups and optimization with response surface methodology. J Mol Liq 221:279–286
Neolaka YAB, Lawa Y, Naat JN, Riwu AAP, Iqbal M, Darmokoesoemo H et al (2020) The adsorption of Cr(VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schleichera oleosa): study of kinetics, isotherms and thermodynamics. J Market Res 9(3):6544–6556
Johnson NN, Yantus ABN, Titus L, Rachmat TTj, Akhmad S, Handoko D and Heri SK, (2021) Adsorption of Cu(II) and Pb(II) using silica@mercapto (HS@M) hybrid adsorbent synthesized from silica of takari sand: optimization of parameters and kinetics. Rasayan J Chem 14(1):550–560
Yantus ABN, Ganden S, Handoko D, Heri SK (2018) Adsorption performance of Cr(VI)-imprinted poly(4-VP-co-MMA) supported on Activated Indonesia (Ende-Flores) natural zeolite structure for Cr(VI) removal from aqueous solution. J Environ Chem Eng 6(2):3436–3443
Yantus ABN, Yosep L, Johnson NN, Arsel APR, Handoko D, Ganden S, Clovia IH, Andrew NA, Heri SK (2020) A Cr(VI)-imprinted-poly(4-VP-co-EGDMA) sorbent prepared using precipitation polymerization and its application for selective adsorptive removal and solid phase extraction of Cr(VI) ions from electroplating industrial wastewater. React Funct Polym 147:104451
Mahreni M, Ramadhan RR, Muhammad FP, Annisa PP, Dini K, Heri SK (2022) Synthesis of Metal Organic Framework (MOF) based Ca Alginate for adsorption of malachite green dye. Polym Bullet 79:11301–11315
Budiana IG, JasmanJ Neolaka YA, Riwu AA, Elmsellem H, Handoko Darmokoesoemo H, Kusuma HS (2021) Synthesis, characterization and application of cinnamoyl C-phenylcalix[4]resorcinarene (CCPCR) for removal of Cr(III) ion from the aquatic environment. J Mol Liq 324:114776
Neolaka YAB, Lawa Y, Naat JN, Pau Riwu AA, Darmokoesoemo H, Supriyanto G et al (2020) A Cr(VI)-imprinted-poly(4-VP-co-EGDMA) sorbent prepared using precipitation polymerization and its application for selective adsorptive removal and solid phase extraction of Cr(VI) ions from electroplating industrial wastewater. React Funct Polym 147:104451
Acknowledgements
This work was supported by the Scientific Research Fund of Young Doctor supported by Qilu Normal University (NO. KYQD20-0012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Liu, X., Zhu, Y. et al. A Zr-based coordination polymer for detection and adsorption of fluoride in water. Polym. Bull. 81, 335–350 (2024). https://doi.org/10.1007/s00289-023-04719-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04719-3