Abstract
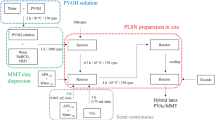
Hybrid adhesives of poly(vinyl acetate) (PVAc) and commercial montmorillonite (MMT) nanoclays, Cloisite® Na (ClNa) and Cloisite® 30B (Cl30B), were synthesized through a semi-continuous surfactant-free emulsion polymerization process. The nanoclay loading was varied based on monomer mass (up to 6 wt %). Ammonium persulfate and sodium bicarbonate were used in all polymerizations as initiator and pH regulator, respectively. Poly(vinyl alcohol) was used as the polymeric stabilizer for this system, with no additional small molecule surfactant added to assist the stabilization of the latexes. The hybrid adhesives were characterized by dynamic light scattering (DLS), X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), dynamic mechanical analysis (DMA) and thermogravimetric analysis (TGA). Results showed that latexes prepared in the presence of both commercial nanoclays exhibited improved colloidal stability. PVAc/ClNa and PVAc/Cl30B hybrid latexes showed reduced viscosity (up to 89% when using ClNa) and coagulum content (up to 75% when using Cl30B) in comparison with pure PVAc latex. The hybrid latexes also exhibited enhanced dynamic mechanical and thermal properties, according to DMA and TGA results. XRD measurements suggested that nanocomposites with exfoliated structures were obtained for PVAc-based adhesives prepared with ClNa (1.5–4.5 wt %) and Cl30B (1.5 wt %), while intercalated nanocomposite was formed when hybrid latexes were prepared with Cl30B at higher loadings (3.0–6.0 wt %). The methodology reported in this study to prepare PVAc/MMT hybrid latexes could be easily scaled-up to industrial conditions allowing for the preparation of adhesives with improved properties.








Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Pavlidou S, Papaspyrides CD (2008) A review on polymer–layered silicate nanocomposites. Prog Polym Sci 33:1119–1198. https://doi.org/10.1016/j.progpolymsci.2008.07.008
Ray SS, Okamoto M (2003) Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci 28:1539–1641. https://doi.org/10.1016/j.progpolymsci.2003.08.002
Alateyah AI, Dhakal HN, Zhang ZY (2013) Processing, properties, and applications of polymer nanocomposites based on layer silicates: a review. Adv Polym Technol 32:1–36. https://doi.org/10.1002/adv.21368
Uddin F (2018) Montmorillonite: An Introduction to Properties and Utilization. In: Zoveidavianpoor Mansoor (ed) Current Topics in the Utilization of Clay in Industrial and Medical Applications. IntechOpen
Strawhecker KE, Manias E (2000) Structure and properties of poly(vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem Mater 12:2943–2949. https://doi.org/10.1021/cm000506g
Alexandre M, Beyer G, Henrist C et al (2001) “One-Pot” preparation of polymer/clay nanocomposites starting from Na+ montmorillonite. 1. melt intercalation of ethylene-vinyl acetate copolymer. Chem Mater 13:3830–3832. https://doi.org/10.1021/cm011095m
Razavi-Nouri M, Sabet A, Mohebbi M (2019) Effect of organoclay content on mechanical and rheological properties of dynamically cross-linked acrylonitrile-butadiene rubber/poly(ethylene-co-vinyl acetate)/organoclay nanocomposites. Polym Bull. https://doi.org/10.1007/s00289-019-03060-y
Fialová L, Capek I, Ianchiş R et al (2008) Kinetics of styrene and butyl acrylate polymerization in anionic microemulsions in presence of layered silicates. Polym J 40:163–170. https://doi.org/10.1295/polymj.PJ2007160
Krikorian V, Pochan DJ (2003) Poly(L-Lactic Acid)/Layered silicate nanocomposite: fabrication, characterization, and properties. Chem Mater 15:4317–4324. https://doi.org/10.1002/app.33633
Fukushima K, Murariu M, Camino G, Dubois P (2010) Effect of expanded graphite/layered-silicate clay on thermal, mechanical and fire retardant properties of poly(lactic acid). Polym Degrad Stab 95:1063–1076. https://doi.org/10.1016/j.polymdegradstab.2010.02.029
Fukushima K, Tabuani D, Camino G (2012) Poly(lactic acid)/clay nanocomposites: effect of nature and content of clay on morphology, thermal and thermo-mechanical properties. Mater Sci Eng C 32:1790–1795. https://doi.org/10.1016/j.msec.2012.04.047
Finnigan B, Martin D, Halley P et al (2004) Morphology and properties of thermoplastic polyurethane composites incorporating hydrophobic layered silicates. Polymer (Guildf) 45:2249–2260. https://doi.org/10.1002/app.21718
Herrera-Alonso JM, Marand E, Little JC, Cox SS (2009) Transport properties in polyurethane/clay nanocomposites as barrier materials: effect of processing conditions. J Memb Sci 337:208–214. https://doi.org/10.1016/j.memsci.2009.03.045
Barick AK, Tripathy DK (2010) Thermal and dynamic mechanical characterization of thermoplastic polyurethane/organoclay nanocomposites prepared by melt compounding. Mater Sci Eng A 527:812–823. https://doi.org/10.1016/j.msea.2009.10.063
Oral A, Tasdelen MA, Demirel AL, Yagci Y (2009) Poly(cyclohexene oxide)/Clay nanocomposites by photoinitiated cationic polymerization via activated monomer mechanism. J Polym Sci Part A Polym Chem 47:5328–5335. https://doi.org/10.1002/pola
González-Vidal N, Muñoz-Guerra S, Martínez de Ilarduya A et al (2010) Poly(hexamethylene terephthalate)-layered silicate nanocomposites. Eur Polym J 46:156–164. https://doi.org/10.1016/j.eurpolymj.2009.10.018
Li X, Kang T, Cho W et al (2001) Preparation and characterization of poly (butyleneterephthalate)/ organoclay nanocomposites. Macromol Rapid Commun 22:1306–1312
Unnikrishnan L, Mohanty S, Nayak SK, Ali A (2011) Preparation and characterization of poly(methyl methacrylate)-clay nanocomposites via melt intercalation: effect of organoclay on thermal, mechanical and flammability properties. Mater Sci Eng A 528:3943–3951. https://doi.org/10.1016/j.msea.2011.01.071
Peeterbroeck S, Alexandre M, Jérôme R, Dubois P (2005) Poly(ethylene-co-vinyl acetate)/clay nanocomposites: effect of clay nature and organic modifiers on morphology, mechanical and thermal properties. Polym Degrad Stab 90:288–294. https://doi.org/10.1016/j.polymdegradstab.2005.03.023
Chien A, Lin K (2007) Morphology and permeability of exfoliated PVAc-MMT nanocomposite films cast from soap-free emulsion-polymerized latices. J Polym Sci Part A Polym Chem 45:5583–5589. https://doi.org/10.1002/pola
Chien A, Lee Y, Lin K (2008) Crosslinkable poly (vinyl acetate)/clay nanocomposite films cast from soap-free emulsion-polymerized latices. J Appl Polym Sci 109:355–362. https://doi.org/10.1002/app
Peruzzo PJ, Bonnefond A, Reyes Y et al (2014) Beneficial in-situ incorporation of nanoclay to waterborne PVAc/PVOH dispersion adhesives for wood applications. Int J Adhes Adhes 48:295–302. https://doi.org/10.1016/j.ijadhadh.2013.09.042
Jung HM, Lee EM, Ji BC et al (2006) Preparation of poly(vinyl acetate)/clay and poly(vinyl acetate)/poly(vinyl alcohol)/clay microspheres. Fibers Polym 7:229–234
Jung HM, Lee EM, Ji BC et al (2007) Poly (vinyl acetate)/poly(vinyl alcohol)/montmorillonite nanocomposite microspheres prepared by suspension polymerization and saponification. Colloid Polym Sci 285:705–710. https://doi.org/10.1007/s00396-006-1623-3
Shi Y, Peterson S, Sogah DY (2007) Surfactant-free method for the synthesis of poly(vinyl acetate) masterbatch nanocomposites as a route to ethylene vinyl acetate/silicate nanocomposites. Chem Mater 19:1552–1564. https://doi.org/10.1021/cm060593y
Corobea MC, Donescu D, Serban S et al (2006) Polymer-nanoclay hybrids obtained by emulsion polymerization of vinyl acetate in the presence of an organically modified montmorillonite. Rev Roum Chim 51:39–46
Donescu D, Corobea MC, Uricanu V et al (2007) Synthesis of polyvinylacetate-sodium montmorillonite hybrids by emulsion polymerization in the presence of anionic surfactants. J Dispers Sci Technol 28:671–679. https://doi.org/10.1080/01932690701341769
Corobea MC, Uricanu V, Donescu D et al (2007) Poly (vinyl acetate)–clay hybrids prepared via emulsion polymerization, assisted by a nonionic surfactant. Mater Chem Phys 103:118–126. https://doi.org/10.1016/j.matchemphys.2007.01.021
Mohsen-Nia M, Doulabi FSM (2011) Synthesis and characterization of polyvinyl acetate/montmorillonite nanocomposite by in situ emulsion polymerization technique. Polym Bull 66:1255–1265. https://doi.org/10.1007/s00289-010-0399-2
Cui H, Du G (2011) Preparation and characterization of PVAc-MMT-DOAB. High Perform Polym 23:40–48. https://doi.org/10.1177/0954008310384288
Cui H, Du G (2013) Influence of synthesis processes on the properties of PVAc-MMT-STAB exfoliated nanocomposites. Polym Plast Technol Eng 52:571–580. https://doi.org/10.1080/03602559.2012.762522
Cui H, Du G (2011) Rheology of PVAc–MMT–STAB. J Adhes Sci Technol 25:1671–1679. https://doi.org/10.1163/016942411X576149
Cui H, Du G (2012) Structure and dynamic mechanical kinetics of polyvinyl acetate-montmorillonite-dioctadecyl dimethyl ammonium bromide. J Thermoplast Compos Mater 26:1206–1222. https://doi.org/10.1177/0892705712439560
Cui H, Du G (2011) Preparation and characterization of exfoliated nano-composite of polyvinyl acetate and montmorillonite. J Chem Eng Mater Sci 2:122–128
Cui H, Du G (2011) Development of an exfoliated nanocomposite prepared by vinyl acetate and organic montmorillonite : structure, dynamic mechanical properties and thermal degradation. Compos Interfaces 18:557–573. https://doi.org/10.1163/156855411X612258
Cui H, Du G (2012) Development of exfoliated nanocomposite prepared from vinyl acetate and montmorillonite. Plast Rubber Compos 41:413–417. https://doi.org/10.1179/1743289812Y.0000000023
Cui H, Du G (2011) Pyrolysis properties of exfoliated nanocomposite synthesized by vinyl acetate and organic montmorillonite through different processes. J Thermoplast Compos Mater 26:156–175. https://doi.org/10.1177/0892705711419878
Cui H, Du G (2012) Preparation and characterization of exfoliated nano-composite prepared by vinyl acetate montmorillonite and dioctadecyl dimethyl ammonium bromide. e-Polymers 2:1–11
Cui H, Fang Q, Du G (2014) Exfoliated nanocomposites of polyvinyl acetate-N-hydroxymethyl acrylamide and intercalated montmorillonite from an octadecyl trimethyl ammonium bromide surfactant. Polym Plast Technol Eng 53:961–968. https://doi.org/10.1080/03602559.2014.886069
Fang Q, Cui H, Du G (2012) Preparation and characterization of PVAc–NMA–MMT. J Thermoplast Compos Mater 26:1393–1406. https://doi.org/10.1177/0892705712461644
Cui H, Fang Q, Du G (2014) Dynamic mechanical and thermal kinetics of polyvinyl acetate type emulsions from vinyl acetate, N-hydroxymethyl acrylamide, and montmorillonite. Adv Polym Technol 33:1–8. https://doi.org/10.1002/adv.21393
Cazotti JC, Salvato RCPJS, Alves GM et al (2019) Effect of clay type on the properties of hybrid latexes of poly(vinyl acetate) and montmorillonite prepared via surfactant-free emulsion polymerization. Polym Bull 76:6305–6325. https://doi.org/10.1007/s00289-019-02708-z
EN 204:2001 (2001) Classification of thermoplastic wood adhesives for non-structural applications. Eur Comm Stand, CEN Brussels
EN 205:2003 (2003) Adhesives wood adhesives for non-structural applications determination of tensile shear strength of lap joints. Eur Comm Stand, Brussels
Messmer NR, Anjos EGR, Guerrini LM, Oliveira MP (2018) Effect of geometry and hybrid adhesive on strength of finger joints of Pinus elliottii subject to humidity and temperature. J Adhes 94:597–614. https://doi.org/10.1080/00218464.2017.1319281
Budhlall BM, Sudol ED, Dimonie VL et al (2001) Role of grafting in the emulsion polymerization of vinyl acetate with poly (vinyl alcohol) as an emulsifier. I. effect of the degree of blockiness on the kinetics and mechanism of grafting. J Polym Sci Part A Polym Chem 39:3633–3654. https://doi.org/10.1002/pola.10016
Carra S, Sliepcevich A, Canevarolo A, Carra S (2005) Grafting and adsorption of poly (vinyl) alcohol in vinyl acetate emulsion polymerization. Polymer (Guildf) 46:1379–1384. https://doi.org/10.1016/j.polymer.2004.11.061
Yamak HB (2013) Emulsion polymerization: effects of polymerization variables on the properties of vinyl acetate based emulsion polymers. In: Yılmaz Faris (ed) Polymer Science. IntechOpen
Xing J, Xu Z, Deng B (2018) Enhanced oxidation resistance of polyphenylene sulfide composites based on montmorillonite modified by benzimidazolium salt. Polymers (Basel) 10:1–15. https://doi.org/10.3390/polym10010083
Morgan AB, Gilman JW (2003) Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and x-ray diffraction: a comparative study. J Appl Polym Sci 87:1329–1338
Moraes RP, Valera TS, Demarquete NR et al (2009) Influence of granulometry and organic treatment of a brazilian montmorillonite on the properties of poly(styrene-co-n-butyl acrylate)/layered silicate nanocomposites prepared by miniemulsion polymerization. J Appl Polym Sci 112:1949–1958
Xi Y, Ding Z, He H, Frost RL (2004) Structure of organoclays - an x-ray diffraction and thermogravimetric analysis study. J Colloid Interface Sci 277:116–120. https://doi.org/10.1016/j.jcis.2004.04.053
Bhattacharya M (2016) Polymer nanocomposites-a comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials (Basel) 9:1–35. https://doi.org/10.3390/ma9040262
Noh MW, Lee DC (1999) Synthesis and characterization of PS-clay nanocomposite by emulsion polymerization. Polym Bull 42:619–626. https://doi.org/10.1007/s002890050510
Blazevska-Gilev J, Spaseska D (2005) Thermal degradation of PVAc. J Univ Chem Technol Metall 40:287–290
Rimez B, Rahier H, Van Assche G et al (2008) The thermal degradation of poly(vinyl acetate) and poly(ethylene-co-vinyl acetate), part I: experimental study of the degradation mechanism. Polym Degrad Stab 93:800–810. https://doi.org/10.1016/j.polymdegradstab.2008.01.010
Kaboorani A, Riedl B (2011) Effects of adding nano-clay on performance of polyvinyl acetate (PVA) as a wood adhesive. Compos Part A Appl Sci Manuf 42:1031–1039. https://doi.org/10.1016/j.compositesa.2011.04.007
Acknowledgements
The authors would like to thank FAPESP for supporting this study. Southern Clay Products, Hexion and Celanese are also greatly thanked for providing the commercial nanoclays, monomer and polymeric stabilizer, respectively, used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cazotti, J.C., Alves, G.M. & Santos, A.M. Surfactant-free hybrid adhesives based on poly(vinyl acetate) and commercial montmorillonite nanoclays. Polym. Bull. 79, 5991–6009 (2022). https://doi.org/10.1007/s00289-021-03787-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03787-7