Abstract
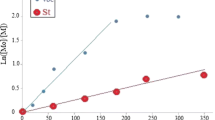
Various random copolymers, poly(styrene-co-ethyl acrylate), were synthesized by free radical bulk copolymerization cocatalyzed by aluminum triflate (Al(OTf)3). The experimental conditions for the polymerization reactions, which include the amount of cocatalyst, polymerization time and ratio of styrene to ethyl acrylate, were investigated. The copolymer molecular weights were determined by gel permeation chromatography coupled to multi-angle laser light scattering. Compositional analysis was performed using proton nuclear magnetic resonance spectrometry. Electron paramagnetic resonance spectroscopy was also used to study the radical species which form in the presence or absence of Al(OTf)3. Kinetic studies were performed by determining monomer conversions as a function of time on gas chromatography. It was found that Al(OTf)3 accelerated the rate of polymerization significantly while also increasing the polymer molecular weights for a given conversion compared to the reactions where the triflate was absent. Al(OTf)3 can be a significantly more cost-effective and abundant alternative polymerization cocatalyst compared to some of the rare lanthanide triflates.















Similar content being viewed by others
References
Chen Y, Sen A (2009) Effect of Lewis acids on reactivity ratios for meth(acrylate)/nonpolar alkene copolymerizations. Macromolecules 42:3951–3957. https://doi.org/10.1021/ma900450p
McManus NT, Penlidis A (1996) A kinetic investigation of styrene/ethyl acrylate copolymerization. J Polym Sci Part A Polym Chem 34:237–248. https://doi.org/10.1002/(SICI)1099-0518(19960130)34:2<237::AID-POLA10>3.0.CO;2-R
Mahdi Abdollahi M, Shahram Mehdipour-Ataei S, Farshid Ziae F (2007) Using 1H-NMR spectroscopy for the kinetic study of the in situ solution free-radical copolymerization of styrene and ethyl acrylate. J Appl Polym Sci 105:2588–2597. https://doi.org/10.1002/app.26290
Corma A, García H (2003) Lewis acids: from conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem Rev 103:4307–4365. https://doi.org/10.1021/cr030680z
Kobayashi S, Nagayama S, Busujima TJ (1998) Lewis acid catalysts stable in water. Correlation between catalytic activity in water and hydrolysis constants and exchange rate constants for substitution of inner-sphere water ligands. Am Chem Soc 120:8287–8288. https://doi.org/10.1021/ja980715q
Koito Y, Nakajima K, Kobayashi H, Hasegawa R, Kitano M, Hara M (2014) Slow reactant-water exchange and high catalytic performance of water-tolerant Lewis acids. Chem Eur J 20:8068–8075. https://doi.org/10.1002/chem.201400240
Amer I, Young DA (2013) Chemically oxidative polymerization of aromatic diamines: the first use of aluminium-triflate as co-catalyst. Polymer 54:505–512. https://doi.org/10.1016/j.polymer.2012.11.078
Amer I, Young DA, Vosloo HCM (2013) Chemical oxidative polymerization of m-phenylenediamine and its derivatives using aluminium triflate as co-catalyst. Eur Polym J 49:3251–3260. https://doi.org/10.1016/j.eurpolymj.2013.06.031
Isobe Y, Fujioka D, Habaue S, Okamoto YJ (2001) Efficient Lewis acid-catalyzed stereocontrolled radical polymerization of acrylamide. Am Chem Soc 123:7180–7181. https://doi.org/10.1021/ja015888l
Nagel M, Poli D, Sen A (2005) Lewis acid-mediated copolymerization of methyl acrylate and methyl methacrylate with 1-alkenes. Macromolecules 38:7262–7265. https://doi.org/10.1021/ma050705r
Luo R, Sen A (2007) Rate enhancement in controlled radical polymerization of acrylates using recyclable heterogeneous Lewis acid. Macromolecules 40:154–156. https://doi.org/10.1021/ma062341o
Isobe Y, Nakano T, Okamoto YJ (2001) Stereocontrol during the free-radical polymerization of methacrylates with Lewis acids. Polym Sci A Polym Chem 39:1463–1471. https://doi.org/10.1002/pola.1123
Amer I, Young DA, Vosloo HCM (2014) Using aluminium triflate as co-catalyst for the polymerization of o-phenylenediamine and its derivatives. Polym Int 63:1229–1237. https://doi.org/10.1002/pi.4628
Lutz JF, Jakubowski W, Matyjaszewski K (2004) Controlled/living radical polymerization of methacrylic monomers in the presence of Lewis acids: influence on tacticity. Macromol Rapid Commun 25:486–492. https://doi.org/10.1002/marc.200300165
Luo R, Chen Y, Sen AJ (2008) Effects of Lewis and Brønsted acids on the homopolymerization of acrylates and their copolymerization with 1-alkenes. Polym Sci A Polym Chem 46:5499–5505. https://doi.org/10.1002/pola.22870
Hadjikyriacou S, Acar M, Faust R (2004) Living and controlled polymerization with alkylaluminum halides as coinitiators. Macromolecules 37:7543–7547. https://doi.org/10.1021/ma049082s
Belleli PG, Ferreira ML, Damiani DE (2000) Addition of Lewis bases and acids. Effects on α-olefins polymerization with soluble metallocenes, 2 propylene. Macromol Chem Phys 201:1466–1475. https://doi.org/10.1002/1521-3935(20000801)201:13<1466::AID-MACP1466>3.0.CO;2-B
Belleli PG, Ferreira ML, Damiani DE (2000) Addition of Lewis bases and acids. Effects on α-olefins polymerization with soluble metallocenes, 1 ethylene. Macromol Chem Phys 201:1458–1465. https://doi.org/10.1002/1521-3935(20000801)201:13<1458::AID-MACP1458>3.0.CO;2-1
Carlson RK, Lee RA, Assam JH, King RA, Nagel ML (2015) Free-radical copolymerisation of acrylamides, acrylates, and α-olefins. Mol Phys 113:1809–1822. https://doi.org/10.1080/00268976.2015.1015641
Román-Leshkov Y, Davis ME (2011) Activation of carbonyl-containing molecules with solid Lewis acids in aqueous media. ACS Catal 1:1566–1580. https://doi.org/10.1021/cs200411d
Fringuelli F, Pizzo F, Vaccaro L (2001) Lewis-acid catalyzed organic reactions in water. The case of AlCl3, TiCl4, and SnCl4 believed to be unusable in aqueous medium. J Org Chem 66:4719–4722. https://doi.org/10.1021/jo010373y
Okuhara T (2002) Water-tolerant solid acid catalysts. Chem Rev 102:3641–3666. https://doi.org/10.1021/cr0103569
Wyatt PJ (1993) Light scattering and the absolute characterization of macromolecules. Anal Chim Acta 272:1–40. https://doi.org/10.1016/0003-2670(93)80373-S
Lee HC, Chang T (1995) On-line determination of dn/dc for size exclusion chromatography coupled with a light scattering detector. Bull Korean Chem Soc 16:640–643
Sage V, Clark JH, Mcquarrie DJ (2004) Supported copper triflate as catalyst for the cationic polymerization of styrene. J Catal 227:502–511. https://doi.org/10.1016/j.jcat.2004.08.013
Yamada B, Kageoka M, Otsu T (1992) ESR study of the radical polymerization of styrene. Macromolecules 25:4828–4831. https://doi.org/10.1007/BF00944835
Kajiwara A (2008) ESR study of the fundamentals of radical. JEOL News 43:39–49
Odian G (2004) Principles of polymerization, 4th edn. Wiley, Hoboken
Tsarevsky NV, Matyjaszewski K (2007) Green atom transfer radical polymerization: from process design to preparation of well-defined environmentally friendly polymeric materials. Chem Rev 107:2270–2299. https://doi.org/10.1021/cr050947p
Fillipov A, Chernikova E, Golubev V, Gryn'ova G, Lin C, Coote ML (2012) Use of spin trap technique for kinetic investigation if elementary steps of RAFT polymerization. In: Kokorin AI (ed) Nitroxides: theory, experiment and applications. Rijeka, Croatia, pp 407–436
Lund A, Danilczuk M (2012) Monomer and polymer radicals of vinyl compounds: EPR and DFT studies of geometric and electronic structures in the adsorbed state. Spectrochim Acta Part A Mol Biomol Spectrosc 98:367–377. https://doi.org/10.1016/j.saa.2012.08.054
Drockenmuller E, Lamps J, Catala J (2004) Living/controlled radical polymerization of ethyl and n-butyl acrylates at 90 °C mediated by sulfinyl nitroxides: influence of the persistent radical stereochemistry. Macromolecules 37:2076–2083. https://doi.org/10.1021/ma0351221
Anseth KS, Anderson KJ, Bowman CN (1996) Radical concentrations, environments, and reactivities during crosslinking polymerizations Macromol. Chem Phys 197:833–848. https://doi.org/10.1002/macp.1996.021970306
Tao Y, He J, Wang Z, Pan J, Jiang H, Chen S, Yang Y (2001) Synthesis of branched polystyrene and poly(styrene-b-4-methoxystyrene) by nitroxyl stable radical controlled polymerization. Macromolecules 34:4742–4748. https://doi.org/10.1002/macp.1996.021970306
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guyo, U., Otto, D.P., Young, D.A. et al. Aluminum triflate-cocatalyzed radical copolymerization of styrene and ethyl acrylate. Polym. Bull. 77, 2227–2247 (2020). https://doi.org/10.1007/s00289-019-02847-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02847-3