Abstract
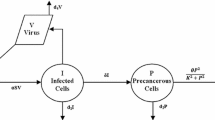
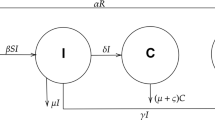
Starting from a deterministic model, we propose and study a stochastic model for human papillomavirus infection and cervical cancer progression. Our analysis shows that the chronic infection state as random variables which have the ergodic invariant probability measure is necessary for progression from infected cell population to cervical cancer cells. It is shown that small progression rate from infected cells to precancerous cells and small microenvironmental noises associated with the progression rate and viral infection help to establish such chronic infection states. It implicates that large environmental noises associated with viral infection and the progression rate in vivo can reduce chronic infection. We further show that there will be a cervical cancer if the noise associated with precancerous cell growth is large enough. In addition, comparable numerical studies for the deterministic model and stochastic model, together with Hopf bifurcations in both deterministic and stochastic systems, highlight our analytical results.






Similar content being viewed by others
References
Arnold L (1998) Random dynamical systems. Springer, New York
Arnold L, Wihstutz V, Eckmann JP, Edited (1990) Lyapunov exponents. In: Proceedings of a conference held in Oberwolfach, May 28, 1990; Lecture notes in mathematics, vol 1486. Springer
Arnold L, Wihstutz V, Edited (1984) Lyapunov exponents. In: Proceedings of a workshop held in Brmen; lecture notes in mathematics, vol 1186. Springer, Berlin, November 12–15, 1984
Asih Tri Sri Noor, Lenhart Suzanne, Wise Steven, Aryati Lina, Adi-Kusumo F, Hardianti Mardiah S, Forde Jonathan (2016) The dynamics of HPV infection and cervical cancer cells. Bull Math Biol 78:4–20
Barnabas RV, Laukkanen P, Koskela P, Kontula O, Lehtinen M, Garnett GP (2006) Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLOS Med 3:0624–0632. https://doi.org/10.1371/journal.pmed.0030138
Bellet LR (2006) Ergodic properties of Markov processes. In: Open quantum systems II. Springer, Berlin, pp 1–39
Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J et al (1995) Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Nat Cancer Inst 87:796–802
Bouvard V et al (2009) A review of human carcinogens-part B: biological agents. Lancet Oncol 10:321–322
Brown VI, White KAJ (2011) The role of optimal control in assessing themost cost-effective implementation of a vaccination programme: HPV as a case study. Math Biosci 231:126–134. https://doi.org/10.1016/j.mbs.2011.02.009123
Bumrungthai S et al (2023) Mathematical modelling of cervical precancerous lesion grade risk scores: linear regression analysis of cellular protein biomarkers and human papillomavirus E6/E7 RNA staining patterns. Diagnostics 13:1084. https://doi.org/10.3390/diagnostics13061084
Butel JS (2000) Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis 21(3):405–26. https://doi.org/10.1093/carcin/21.3.405
Clifford GM, Smith JS, Aguado T, Franceschi S (2003) Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 89(101–105):6601024. https://doi.org/10.1038/sj.bjc
de Villiers EM (1994) Human pathogenic papillomavirus types: an update. Curr Top Microbiol Immunol 184:1–12
Dieu NT, Du NH, Nguyen HD, Yin G (2016) Protection zones for survival of species in random environment. SIAM J Appl Math 76(4):1382–1402
Du NH, Nguyen HD, Yin G (2016) Conditions for permanence and ergodicity of certain stochastic predator-prey models. J Appl Probab 53:187–202
Duan J (2015) An introduction to stochastic dynamics. Cambridge University Press, Cambridge
Elbasha EH (2008) Global stability of equilibria in a two-sex HPV vaccination model. Bull Math Biol 70:894–909. https://doi.org/10.1007/s11538-007-9283-0
Frazer IH, Cox JT, Mayezux EJ, Franco EL, Moscicki AB, Palefsky JM et al (2006) Advances in prevention of cervical cancer and other human papillomavirus-related diseases. Pediat Infect Dis J 25:S65–S81
Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ (2008) Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst 100:308–320. https://doi.org/10.1093/jnci/djn019
Graham SV (2017) The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci 131:2201–2221. https://doi.org/10.1042/CS20160786
Hassard BD, Kazarinoff ND, Wan Y-H (1981) Theory and applications of Hopf Bifurcation. Cambridge University Press, Cambridge
Hausen ZH (2001) Oncogenic DNA viruses. Oncogene 20:7820–7823
Hening A, Nguyen HD (2018) Coexistence and extinction for stochastic Kolmogorov systems. Ann Appl Probab 28:1893–1942
Herfs M et al (2012) A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. PNAS 109(26):10516–10521
Hörmander L (1967) Hypoelliptic second order differential equations. Acta Math 119:147–171
Ikeda N, Watanabe S (1989) Stochastic differential equations and diffusion processes, 2nd edn. North-Holland, Amsterdam
Iskandar R, Taghavi K, Low N, Bramer WM, Egger M, Rohner E (2022) Mathematical models for evaluating effectiveness and cost-effectiveness of cervical cancer control policies in populations including women living with human immunodeficiency virus: a scoping review. Value Health Reg Issues 32:39–46
Jorgensen B (1982) Statistical property of the generalized inverse Gaussian distribution. Springer, New York
Jurdjevic V (1996) Geometric control theory. Cambridge University Press, Cambridge
Keller H (1996) Random attractors and and bifurcations of the stochastic Lorenz system. Technical Report 389. Institut für Dynamische Systeme, Universität Bremen
Khasminskii R (2012) Stochastic stability of differential equations. In: Stochastic modeling and applied probability, 2nd edn
Kim JJ, Brisson M, Edmunds WJ, Goldie SJ (2008) Modeling cervical cancer prevention in developed countries. Vaccine 26:K76–K86. https://doi.org/10.1016/j.vaccine.2008.06.009
Lee C, Laimins LA (2007) The differentiation-dependent life cycle of human papillomaviruses in keratinocytes. In: Garcea R, Di Maio D (eds) The papillomaviruses. Springer, New York, pp 45–67
Lee SL, Tameru AM (2012) A mathematical model of human papillomavirus (HPV) in the United States and its impact on cervical cancer. J Cancer 3:262–268. https://doi.org/10.7150/jca.4161
Mao X (1997) Stochastic differential equations and their applications. Horwood Publishing, Chichester
Meyn SP, Tweedie RL (1993) Stability of Markovian processes II: continuous-time processes and sampled chains. Adv Appl Probab 25:487–517
Meyn SP, Tweedie RL (1993) Stability of Markovian processes III: Foster–Lyapunov criteria for continuous-time processes. Adv Appl Probab 25:518–548
Munoz N, Castellsague X, de Gonzalez AB, Gissmann L (2006) Chapter 1: HPV in the etiology of human cancer. Vaccine 24:S1–S10
Murtono M, Ndii MZ, Sugiyanto S (2019) Mathematical model of cervical cancer treatment using chemotherapy drug. Biol Med Nat Prod Chem 8(1):11–15. https://doi.org/10.14421/biomedich.2019.81.11-15
Nualart D (2006) The Malliavin calculus and related topics. Springer, Berlin
Ostor AG (1993) Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 12:186–192
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118:3030–3044
Phan TA, Tian JP (2020) Basic stochastic model for tumor virotherapy. Math Biosci Eng 17(4):4271–4294
Phan TA, Tian JP (2022) Hopf bifurcation without parameters in deterministic and stochastic modeling of cancer virotherapy, part II. J Math Anal Appl 515:126444. https://doi.org/10.1016/j.jmaa.2022.126444
Phan TA, Nguyen HD, Tian JP (2021) Deterministic and stochastic modeling for PDGF-driven gliomas reveals a classification of gliomas. J Math Biol 83:22
Phan TA, Tian JP, Wang B (2021) Dynamics of cholera epidemic models in fluctuating environments. Stoch Dyn 21(02):2150011. https://doi.org/10.1142/S0219493721500118
Plummer M et al (2016) Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 4:e609–e616
Reingold AL (2000) Infectious Disease Epidemiology in the 21st Century: Will It Be Eradicated or Will It Reemerge? Epidemiol Rev 22:57–63
Ryser MD, Gravittc PE, Myersd ER (2017) Mechanistic mathematical models: an underused platform for HPV research. Papillomavirus Res 3:46–49
Shah KV, Howley PM (1996) Papillomavirus. In: Fields BN, Knipe DM, Howley PM (eds) Virology, 3rd edn. Lippincott-Raven Press Ltd, New York, pp 2077–2109
Sierra-Rojas JC, Reyes-Carreto R, Vargas-De-Leon C, Camacho JF (2022) Modeling and mathematical analysis of the dynamics of HPV in cervical epithelial cells: transient, acute, latency, and chronic infections. Comput Math Methods Med Article ID 8650071. https://doi.org/10.1155/2022/8650071
White MK, Pagano JS, Khalili K (2014) Viruses and human cancers: a long road of discovery of molecular paradigms. Clin Microbiol Rev 27(3):463–81. https://doi.org/10.1128/CMR.00124-13
Wright TC, Ferenczy A (2002) Anatomy and histology of the cervix, Blaustein’s pathology of the female genital tract, 5th edn. Springer, New York, pp 207–224
Zapatka M, Borozan I, Brewer DS, Iskar M, Grundhoff A, Alawi M et al (2020) The landscape of viral associations in human cancers. Nat Genet 52:320–330
Ziyadi N (2017) A male–female mathematical model of human papillomavirus (HPV) in African American population. Math Biol Eng 14(1):339–358. https://doi.org/10.3934/mbe.2017022
Acknowledgements
TAP would like to acknowledge support by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number P20GM104420. JPT would like to acknowledge grant National Institutes of Health (U54CA132383) which supported FS for a summer during the grant periods. We thank two anonymous reviewers for their constructive suggestions which help to improve the presentation of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
To comprehend how environmental noises and randomness affect the dynamical behaviors of the deterministic system (4), we need to study how the threshold \(\lambda \) is related to the reproduction number \(R_0\). Since \(R_0=\frac{\alpha n}{e(a+\delta )}\) is a decreasing function of the parameter \(\delta \), which is the progression rate from infected cells to precancerous cells, we can consider the threshold \(\lambda \) as a function of \(\delta \) as well as a function of noise intensities. Propositions 2.1 and 2.2 give the behavior of the threshold \(\lambda \) with respect to system parameters and noise intensities. Because Proposition 2.2 is a direct consequence of Proposition 2.1, so we only present the proof of Proposition 2.1
Proof of Proposition 2.1
Since \(\dfrac{\Theta }{w}=\dfrac{a+\delta -e}{2\sqrt{\alpha n}}+\dfrac{\tau _1^2-\tau _2^2}{4\sqrt{\alpha n}}\), the threshold \(\lambda \) can be rewritten as
Let \(D_{\Theta }(w):=\dfrac{K_{\Theta +1}(w)K_{\Theta -1}(w)}{K^2_{\Theta }(w)}\) where \(K_{\Theta }(\cdot )\) is the modified Bessel function of third kind with index \(\Theta \). From Jorgensen (1982) in p. 172 and p. 175, we have \(D_{\Theta }(w)=R_{\Theta }(w)R_{-\Theta }(w)\quad \text {and}\quad D_{\Theta }(w)=R_{\Theta }(w)\left[ R_{\Theta }(w)-2\dfrac{\Theta }{w}\right] \). Hence
Since \(w>0\), a result in p. 173 in Jorgensen (1982) implies that \(R_{\Theta }(w)\) is an increasing function of \(\Theta \) and thus \(\lambda \) is a decreasing function of \(\Theta \). But, as \(\Theta \) is an increasing function of \(\delta \), so \(\lambda \) is a decreasing function of \(\delta \). Since \(R_0\) is a decreasing function of \(\delta \), \(\lambda \) is an increasing function of \(R_0\).
Next, since \({\overline{\lambda }}=\lim \limits _{(\tau _1,\tau _2)\rightarrow (0,0)}\lambda \) and \(R_{\Theta }(w)=\dfrac{\Theta }{w}+\sqrt{\left( \dfrac{\Theta }{w}\right) ^2+D_{\Theta }(w)}\),
As \(\dfrac{\Theta }{w}\rightarrow \dfrac{a+\delta -e}{2\sqrt{\alpha n}}\) and \(D_{\Theta }(w)\rightarrow 1\) as \((\tau _1,\tau _2)\rightarrow (0,0)\), so
It implies that \({\overline{\lambda }}=0\) iff \(R_0=1\), \({\overline{\lambda }}<0\) iff \(R_0<1\), and \({\overline{\lambda }}>0\) iff \(R_0>1\). This completes the proof. \(\square \)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Phan, T.A., Sarower, F., Duan, J. et al. Stochastic dynamics of human papillomavirus delineates cervical cancer progression. J. Math. Biol. 87, 85 (2023). https://doi.org/10.1007/s00285-023-02018-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-023-02018-z
Keywords
- Chronic infection state
- Ergodic invariant probability measure
- Cervical cancer
- Stochastic dynamical bifurcation