Abstract
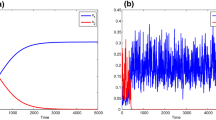
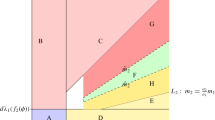
In this work, we consider a model of two microbial species in a chemostat in which one of the competitors can produce a toxin (allelopathic agent) against the other competitor, and is itself inhibited by the substrate. The existence and stability conditions of all steady states of the reduced model in the plane are determined according to the operating parameters. With Michaelis-Menten or Monod growth functions, it is well known that the model can have a unique positive equilibrium which is unstable as long as it exists. By including both monotone and non-monotone growth functions (which is the case when there is substrate inhibition), it is shown that a new positive equilibrium point exists which can be stable according to the operating parameters of the system. This general model exhibits a rich behavior with the coexistence of two microbial species, the multi-stability, the occurrence of stable limit cycles through super-critical Hopf bifurcations and the saddle-node bifurcation of limit cycles. Moreover, the operating diagram describes some asymptotic behavior of this model by varying the operating parameters and illustrates the effect of the inhibition on the emergence of the coexistence region of the species.






Similar content being viewed by others
References
Abdellatif N, Fekih-Salem R, Sari T (2016) Competition for a single resource and coexistence of several species in the chemostat. Math Biosci Eng 13:631–652
Andrews JF (1968) A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol Bioeng 10:707–723
Bar B, Sari T (2020) The operating diagram for a model of competition in a chemostat with an external lethal inhibitor. Discrete Contin Dyn Syst-Series B 25:2093–21203
Bernard O, Hadj-Sadock Z, Dochain D, Genovesi A, Steyer JP (2001) Dynamical model development and parameter identification for an anaerobic wastewater treatment process. Biotechnol Bioeng 75:424–438
Butler GJ, Wolkowicz GSK (1985) A mathematical model of the chemostat with a general class of functions describing nutrient uptake. SIAM J Appl Math 45:138–151
De Freitas MJ, Fredrickson AG (1978) Inhibition as a factor in the maintenance of the diversity of microbial ecosystems. J General Microbiol 106:307–320
De Leenheer P, Li B, Smith HL (2003) Competition in the chemostat: some remarks. Can Appl Math Q 11:229–248
Dellal M, Bar B (2021) Global analysis of a model of competition in the chemostat with internal inhibitor. Discrete Contin Dyn Syst-Series B 26:1129–1148
Dellal M, Lakrib M, Sari T (2018) The operating diagram of a model of two competitors in a chemostat with an external inhibitor. Math Biosci 302:27–45
Dellal M, Bar B, Lakrib M (2022) A competition model in the chemostat with allelopathy and substrate inhibition. Discrete Contin Dyn Syst-Series B 27:2025–2050
El Hajji M, Mazenc F, Harmand J (2010) A mathematical study of a syntrophic relationship of a model of anaerobic digestion process. Math Biosci Eng 7:641–656
Fekih-Salem R, Rapaport A, Sari T (2016) Emergence of coexistence and limit cycles in the chemostat model with flocculation for a general class of functional responses. Appl Math Modell 40:7656–7677
Fergola P, Cerasuolo M, Pollio A, Pinto G, Della Grecac M (2007) Allelopathy and competition between chlorella vulgaris and pseudokirchneriella subcapitata: experiments and mathematical model. Ecol Modell 208:205–214
Fgaier H, Kalmokoff M, Ells T, Eberl HJ (2014) An allelopathy based model for the listeria overgrowth phenomenon. Math Biosci 257:13–26
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297
Harmand J, Lobry C, Rapaport A, Sari T (2017) The chemostat: mathematical theory of microorganism cultures. Wiley-ISTE, London
Hsu SB, Waltman P (1998) Competition in the chemostat when one competitor produces a toxin. Jpn J Ind Appl Math 15:471–490
Hsu SB, Waltman P (2004) A survey of mathematical models of competition with an inhibitor. Math Biosci 187:53–91
Hsu SB, Li YS, Waltman P (2000) Competition in the presence of a lethal external inhibitor. Math Biosci 177:177–199
Kuznetsov Y (2004) Elements of applied bifurcation theory. Applied mathematical sciences. Springer, New York
Lenski RE, Hattingh S (1986) Coexistence of two competitors on one resource and one inhibitor: a chemostat model based on bacteria and antibiotics. J Theor Biol 122:83–93
Li B (1998) Global asymptotic behavior of the chemostat: general response functions and different removal rates. SIAM J Appl Math 59:411–422
Martines IP, Kojouharov HV, Grover JP (2009) A chemostat model of resource competition and allelopathy. Appl Math Comput 215:573–582
Monod J (1950) La technique de culture continue: théorie et applications. Ann de l’Institut Pasteur 79:390–410
Mtar T, Fekih-Salem R, Sari T (2022) Mortality can produce limit cycles in density-dependent models with a predator-prey relationship. Discrete Contin Dyn Syst-Series B 27:7445–7467
Nouaoura S, Fekih-Salem R, Abdellatif N, Sari T (2022) Operating diagrams for a three-tiered microbial food web in the chemostat. J Math Biol 85:44
Pavlou S (1999) Computing operating diagrams of bioreactors. J Biotechnol 71:7–16
Sari T (2013) Competitive exclusion for chemostat equations with variable yields. Acta Appl Math 123:201–219
Sari T, Mazenc F (2011) Global dynamics of the chemostat with different removal rates and variable yields. Math Biosci Eng 8:827–840
Smith HL, Tang B (1989) Competition in the gradostat: the role of the communication rate. J Math Biol 27:139–165
Smith HL, Waltman P (1995) The theory of the chemostat: dynamics of microbial competition. Cambridge University Press, Cambridge
Sobieszek S, Wolkowicz GSK, Wade MJ (2020) Rich dynamics of a three-tiered anaerobic food-web in a chemostat with multiple substrate inflow. Math Biosci Eng 17:7045–7073
Wade MJ, Harmand J, Benyahia B, Bouchez T, Chaillou S, Cloez B, Godon J, Boudjemaa BM, Rapaport A, Sari T, Arditi R, Lobry C (2016) Perspectives in mathematical modelling for microbial ecology. Ecol Modell 321:64–74
Weedermann M, Seo G, Wolkowicz G (2013) Mathematical model of anaerobic digestion in a chemostat: effects of syntrophy and inhibition. J Biol Dyn 7:59–85
Acknowledgements
The authors would like to thank the anonymous referees very much for their valuable comments and suggestions. This work has been supported by the ”Directorate General for Scientific Research and Technological Development, Ministry of Higher Education and Scientific Research, Algeria” and the Euro-Mediterranean research network TREASURE (http://www.inra.fr/treasure).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A Proofs
A Proofs
1.1 A.1 Proof of Proposition 2
Equilibria of (8) are the solutions of the set of Eq. (9).
-
We first note that if \(x= 0\) and \(y= 0\), we obtain the washout equilibrium \(E_0=(0,0)\) which always exists.
-
If \(y = 0\) and \(x>0\), then from the first equation of (9), we have \(f_1\left( S^{0}-x_1\right) =D \), or equivalently \(S^{0}-x_1=\lambda _1\), where \(\lambda _1\) is given by (4). Thus, \(x_1=S^{0}-\lambda _1\). This equilibrium exists if and only if \(x_1>0\); that is, \(\lambda _1<S^{0}\).
-
If \(y>0\) and \(x=0\), then from the second equation of (9), we have
$$\begin{aligned} (1-k)f_2\left( S^{0}-(1+c)y_2\right) =D, \end{aligned}$$where \(c=\frac{k}{1-k}\). From hypothesis (H2), equation \((1-k)f_2\left( S\right) =D\) has two positive solutions \(S^1=\lambda _2\) and \(S^2=\mu _2\) (with \(\lambda _2\le \mu _2\)) or no solution and we then put \(\lambda _2=+\infty \) and \(\mu _2=+\infty \). It follows that
$$\begin{aligned} y_2^1=(S^{0}-\lambda _2)(1-k) \ \ \ \ \hbox {and} \ \ \ \ y_2^2=(S^{0}-\mu _2)(1-k). \end{aligned}$$(16)Thus, one can conclude that equilibrium \({E}_2^1\) exists if and only if \(y_2^1>0\); that is, \(\lambda _2<S^{0}\), and \({E}_2^2\) exists if and only if \(y_2^2>0\); that is, \(\mu _2<S^{0}\).
-
If \(y>0\) and \(x>0\), then from the second equation of (9), we have
$$\begin{aligned} S^{0}-x_{c_1}-(1+c)y_{c_1}=\lambda _2 \ \ \ \ \hbox {and} \ \ \ \ S^{0}-x_{c_2}-(1+c)y_{c_2}=\mu _2, \end{aligned}$$and from the first equation, we get
$$\begin{aligned} y_{c_1}=\dfrac{1-k}{ k}f^{-1}\left( \frac{D}{f_1(\lambda _2)}\right) \ \ \ \ \hbox {and} \ \ \ \ y_{c_2}=\dfrac{1-k}{ k}f^{-1}\left( \frac{D}{f_1(\mu _2)}\right) . \end{aligned}$$Therefore \(y_{c_j}\) and \(x_{c_j}\) are given by (10) and (11). Hence, a positive equilibrium \(E_c^j\), \(j=1,2\), of system (8), if it exists, is unique. Let us study conditions of existence of \(E_c^j\). Using the fact that f is decreasing and \(f(0)=1\) (hypothesis (H3)) we have
$$\begin{aligned} y_{c_j}>0\Longleftrightarrow & {} D<f_{1}(S^{j}) \Longleftrightarrow \lambda _1<S^{j} \end{aligned}$$(17)and
$$\begin{aligned} x_{c_j}>0\Longleftrightarrow & {} S^0-S^{j}-(1+c)y_{c_j}>0 \nonumber \\\Longleftrightarrow & {} S^0>S^{j}+\dfrac{1}{ k}f^{-1}\left( \frac{D}{f_1(S^{j})}\right) \nonumber \\\Longleftrightarrow & {} S^0>F_j(D). \end{aligned}$$(18)From (17) and (18), we conclude finally that \(E_c^j\) exists if and only if \(\lambda _1<S^{0}\) and \(S^0>F_j(D)\). \(\square \)
1.2 A.2 Proof of Proposition 3
-
At washout equilibrium \(E_0\), the matrix M defined by (12) is
$$\begin{aligned}M_0= \left[ \begin{array}{cc} f_1(S^0)-D &{} 0\\ 0 &{} (1-k)f_2(S^0)-D \end{array} \right] . \end{aligned}$$The eigenvalues of \(M_0\) are: \(f_1(S^0)-D\) and \((1-k)f_2(S^0)-D\). Then, equilibrium \(E_0\) is LES if and only if \(f_1(S^0)<D\) and \((1-k)f_2(S^0)<D\).
-
Suppose that equilibrium \(E_1\) exists; that is, \(\lambda _1\!<\!S^{0}\). At \(E_1\) the matrix M defined by (12) is
$$\begin{aligned}M_1= \left[ \begin{array}{cc} -(S^{0}-\lambda _1)f_1'(\lambda _1) &{} A\\ 0 &{} (1-k)f_2(\lambda _1)-D \end{array} \right] , \end{aligned}$$where \(A=(cf'(0)f_1(\lambda _1)-(1+c)f_1'(\lambda _1))(S^{0}-\lambda _1)\). The eigenvalues of \(M_1\) are: \(-(S^{0}-\lambda _1)f_1'(\lambda _1)<0\) and \((1-k)f_2(\lambda _1)-D\). Therefore, \(E_1\) is LES if and only if \((1-k)f_2(\lambda _1)<D\), or equivalently, \(\lambda _1<\lambda _2\) or \(\lambda _1>\mu _2\).
-
Suppose that equilibria \(E_2^j\), \(j\!=\!1,2\), exist; that is, \(\lambda _2\!<\!S^{0}\) and \(\mu _2\!<\!S^{0}\). At \(E_2^j\) the matrix M defined by (12) is
$$\begin{aligned}M_2= \left[ \begin{array}{cc} f(k(S^{0}-S^j))f_1(S^j)-D &{} 0\\ -(1-k)y^j_2f_2'(S^j) &{} -y^j_2f_2'(S^j) \end{array} \right] . \end{aligned}$$The eigenvalues of \(M_2\) are: \(-y^j_2f_2'(S^j) \) and \(f(k(S^{0}-S^j))f_1(S^j)-D\). At \(E_2^1\), we have \(-y^j_2f_2'(S^j)<0\) because \(f'_2(S^1)=f'_2(\lambda _2)>0\). Therefore, \(E_2^1\) is LES if and only if \(f(k(S^{0}-\lambda _2))f_1(\lambda _2)<D\). At \(E_2^2\), we have \(-y^j_2f_2'(S^j)>0\) because \(f'_2(S^2)=f'_2(\mu _2)<0\). Thus, \(E_2^2\) is unstable.
-
At \(E_c^j\), the matrix M defined by (12) takes the form
$$\begin{aligned}M_c= \left[ \begin{array}{cc} m_{11} &{} m_{12} \\ m_{21} &{} m_{22} \\ \end{array} \right] , \end{aligned}$$where
$$\begin{aligned} m_{11}= & {} -f\left( cy_{c_j}\right) f'_1\left( S^{j}\right) x_{c_j}, \\ m_{12}= & {} cf'\left( cy_{c_j}\right) f_1\left( S^{j}\right) x_{c_j}-(1+c)f\left( cy_{c_j}\right) f'_1\left( S^{j}\right) x_{c_j}, \\ m_{21}= & {} -(1-k) f'_2\left( S^{j}\right) y_{c_j}\quad \text {and}\quad m_{22}=- f'_2\left( S^{j}\right) y_{c_j}. \end{aligned}$$At \(E_c^1\), we have
$$\begin{aligned} \det (M_c)=k f'_2(S^{1})f'( cy_{c_1}) f_1( S^{1})x_{c_1}y_{c_1} <0 \end{aligned}$$because \(f'_2(S^{1})=f'_2(\lambda _2)>0\). Thus, \(E_c^1\) is unstable. At \(E_c^2\), we have
$$\begin{aligned} \det (M_c)=k f'_2(S^{2})f'(cy_{c_2}) f_1( S^{2})x_{c_2}y_{c_2}>0 \end{aligned}$$because \(f'_2(S^{2})=f'_2(\mu _2)<0\). Therefore, \(E_c^2\) is LES if and only if
$$\begin{aligned} tr(M_c)=-f( cy_{c_2}) f'_1(\mu _2)x_{c_2}- f'_2(\mu _2)y_{c_2}<0, \end{aligned}$$or equivalently,
$$\begin{aligned} Df'_1(\mu _2)x_{c_2}+f_1(\mu _2) f'_2(\mu _2)y_{c_2}>0. \end{aligned}$$\(\square \)
1.3 A.3 Proof of Lemma 1
Using definitions (11) of \(x_{c_2}\) and \(y_{c_2}\), one has
\(\square \)
1.4 A.4 Proof of Theorem 3.1
The proof uses ideas in Sobieszek et al. (2020). The characteristic polynomial at \(E_c^2\) takes the form
The fact that \(C\ne 0\) means that the only way that \(E_c^2\) is destabilized is for the two complex conjugate eigenvalues \(\lambda _j(D)=\alpha (D)\pm i\beta (D)\), \(j = 1,2\), to cross the imaginary axis (because of the continuity of coefficients of F with respect to the parameters), which is the case when
To have a simple Hopf bifurcation, we need the transversality condition to be verified, i.e. the pair of complex conjugate eigenvalues cross the imaginary axis with non-zero speed. This is guaranteed because the derivative of \(F_3\) with respect to \(S^0\) is not equal to zero when \(F_3(D,S^0)=0\).
Here, \(\partial _{S^0} F_3(D,S^0)=Df'_1(\mu _2) \partial _{S^0} x_{c_2}(D,S^0)=Df'_1(\mu _2)>0,\) where \(x_{c_2}\) is given by (11). \(\square \)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zitouni, N.E.H., Dellal, M. & Lakrib, M. Substrate inhibition can produce coexistence and limit cycles in the chemostat model with allelopathy. J. Math. Biol. 87, 7 (2023). https://doi.org/10.1007/s00285-023-01943-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-023-01943-3