Abstract
Bone remodelling is a fundamental biological process that controls bone microrepair, adaptation to environmental loads and calcium regulation among other important processes. It is not surprising that bone remodelling has been subject of intensive both experimental and theoretical research. In particular, many mathematical models have been developed in the last decades focusing in particular aspects of this complicated phenomenon where mechanics, biochemistry and cell processes strongly interact. In this paper, we present a new model that combines most of these essential aspects in bone remodelling with especial focus on the effect of the mechanical environment into the biochemical control of bone adaptation mainly associated to the well known RANKL-RANK-OPG pathway. The predicted results show a good correspondence with experimental and clinical findings. For example, our results indicate that trabecular bone is more severely affected both in disuse and disease than cortical bone what has been observed in osteoporotic bones. In future, the methodology proposed would help to new therapeutic strategies following the evolution of bone tissue distribution in osteoporotic patients.













Similar content being viewed by others
Notes
In the biochemical model (Klika and Maršík 2010), the mechanical stimulation has different impacts on different considered reactions in order to reflect both inhibitory (e.g. osteoclastogenesis is inhibited by mechanical stimulation) and stimulatory (bone remodelling is stimulated overall by mechanical loading) effects of mechanical stimulation. That is why there are more mechanical parameters \(\mathcal {D}_{\alpha }\).
The presented model can be extended with further details of biochemical control once considered necessary or advantageous.
Abbreviations
- \(v_b\) :
-
Bone volume fraction [1]
- \(v_m\) :
-
Mineral volume fraction of bone [1]
- \(v_o\) :
-
Organic volume fraction of bone [1]
- \(v_v\) :
-
Void volume fraction [1]
- \(dam\) :
-
Damage concentration [1]
- \(r_{\alpha }\) :
-
Rate of \(\alpha \)-th interaction \(\left[ \text{ kmol }\cdot \text{ m }^{-3}\cdot \text{ s }^{-1}\right] \)
- \(\mathcal {A}_{\alpha }\) :
-
Affinity of \(\alpha \)-th reaction \(\left[ \text{ J }\cdot \text{ kmol }^{-1}\right] \)
-
 :
: -
The deformation rate tensor
 and its j-\(th\) invariant; \(j=1\) and \(j=2\) represent rate of volume dilatation and shear rate, respectively [\(s^{-1}\)]
and its j-\(th\) invariant; \(j=1\) and \(j=2\) represent rate of volume dilatation and shear rate, respectively [\(s^{-1}\)] - \({\varvec{\varepsilon }},~\varepsilon ^{(j)}\) :
-
The deformation tensor \(\varepsilon \) and its j-\(th\) invariant [1]
- \(l_{v\alpha },~l_{\alpha \alpha }\) :
-
Phenomenological constants in classical irreversible thermodynamics (CIT)
- \(C_j, [\mathrm{C_j}]\) :
-
j-\(th\) substance and its molar concentration \(\left[ \text{ kmol }\cdot \text{ m }^{-3}\right] \)
- \(\nu _{j\alpha },~\nu '_{j\alpha }\) :
-
Stoichiometric coefficients of substance \(C_j\) entering \(\alpha \)-th reaction or being produced in it (denoted with a prime), respectively [1]
- \(k_{+\alpha },~k_{-\alpha }\) :
-
Forward and backward reaction rate constant of \(\alpha \)-th reaction
- \(\alpha \) :
-
Ash fraction [1]
- \(\nu \) :
-
Poisson ratio [1]
- \(E\) :
-
Young’s modulus \(\left[ \text{ M } \text{ kg }\cdot \text{ m }^{-1}\cdot \text{ s }^{-1}\right] \)
- \(\xi \) :
-
Daily strain history [1]
- \(L\) :
-
Number of loading cases [1]
- \(N_i\) :
-
Number of cycles of i-\(th\) loading case [1]
- \(\bar{\varepsilon }\) :
-
Strain level [1]
- \(\bar{d}\) :
-
Strain rate level [\(s^{-1}\)]
- \(U\) :
-
Strain energy density \(\left[ \text{ kg }\cdot \text{ m }^{-1}\cdot \text{ s }^{-2}\right] \)
- \(f_i\) :
-
Frequency of a considered loading case [\(s^{-1}\)]
- \(T_i\) :
-
Period of a considered loading case [s]
- \(\mathcal {D}_{\alpha }\) :
-
Parameters describing the influence of mechanical stimulus on \(\alpha -th\) interaction [1]
- \(A\) :
-
Parameter used for relating \(\mathcal {D}_{\alpha }\) to \(\xi \) [1]
- \(\mathcal {D}_{\alpha ,ref},~\xi _{ref}\) :
-
Reference values of mechanical stimulus [1]
- \(\varepsilon _{crit}\) :
-
Critical strain value of bone tissue [1]
- \(N_f\) :
-
Fatigue life expectation (number of cycles) [1]
- \(K_i\) :
-
(\(i=t,c\)) Constant of proportionality in fatigue life expectation [1]
- \(\delta _i\) :
-
(\(i=t,c\)) Exponent in fatigue life expectation [1]
- \(dam_i\) :
-
(\(i=t,c\)) Damage in trabecular or cortical bone [1]
- \(C_{c,t_1,t_2},~\gamma _{c,t}\) :
-
Fitted parameters in evolution laws for damage growth [1]
- \(\varepsilon _u\) :
-
Ultimate tensile strain, function of calcium content [1]
- \(v_{b,res}\) :
-
Amount of resorbed bone volume fraction [1]
- \(\rho _{res}\) :
-
Difference in bone tissue density caused by resorption [kg\(\cdot \text{ m }^{-3}\)]
- \(\rho _{max}\) :
-
Maximal amount of bone density (true bone density) [kg\(\cdot \text{ m }^{-3}\)]
- \(J_{Old\_B}\) :
-
Normalised turnover rate in equilibrium [1]
- \(n_{C_j}\) :
-
Normalised concentration of \(j\)-th substance [1]
- \({n_{Old\_B}}_{,max}\) :
-
Maximal possible value of \(n_{Old\_B}\) corresponding to intact bone tissue under the maximal mechanical stimulus \(\xi (\varepsilon _{crit})\) [1]
- \(m_m,~m_o\) :
-
Mineral/organic mass [kg\(\cdot \text{ m }^{-3}\)]
- \(\rho _m,~\rho _o\) :
-
Mineral/organic true densities [kg\(\cdot \text{ m }^{-3}\)]
- \(v_m^{\mathrm{secondary}}\) :
-
Exponential law for secondary mineralisation [1]
- \(v_m^{prim}\) :
-
Mineral volume fraction value at the end of the primary phase [1]
- \(v_j^0\) :
-
Initial value of \(v_j,~j=m,b,o\) [1]
- \(\tau _{BR}\) :
-
Time corresponding to the end of the primary phase [1]
- \(v_m^{max}\) :
-
\(v_m\) corresponding to the maximal mineral content found in bone [1]
- \(\kappa \) :
-
Exponent in secondary mineralisation phase [1]
- \(v_m^{\mathrm{OldB}}\) :
-
Average mineral content of old bone \(Old\_B\) [1]
- \(v_m^{\mathrm{NewB}}\) :
-
Average mineral content of new bone \(New\_B\) [1]
- \(n_{prim}\) :
-
Fraction of bone undergoing primary mineralisation [1]
- \(X^0\) :
-
Initial value of \(X\) (e.g. \(v_b^0,\alpha ^0, [\mathrm{PTH}]_0\))
- \(BT_{ind}\) :
-
Bone tissue index (a measure of total bone mass) [\(\text{ m }^3\)]
- \(BMD_{ind}\) :
-
Bone mineral index (a measure of total mineral mass) [kg]
- \(BMD(i)\) :
-
Bone mineral density at \(i\)-th element [kg\(\cdot \text{ m }^{-3}\)]
- \(V(i)\) :
-
Volume of the \(i\)-th element [\(\text{ m }^3\)]
- \(n_{C_{j,0}}\) :
-
Initial normalised concentration of j-\(th\) substance [1]
- \([\mathrm{C_j}]_{st}\) :
-
Standard concentration of j-\(th\) substance [1]
- \(n_{C_j,st}\) :
-
Normalised equivalent standard concentration of j-\(th\) substance [1]
- \(n_{i_0}^{RRO,C_j}\) :
-
\(C_j \in \{PTH,~estr,~NO\}\), \(i \in \{RKL,~OPG\}\); predicted initial value of \(n_i\) (from a submodel for \(C_j\) influence on RANKL-RANK-OPG pathway) used as input in the RANKL-RANK-OPG model [1]
References
Aubin JE, Bonnelye E (2000) Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Medscape Womens Heal 5(2):5
Beaupre GS, Orr TE, Carter DR (1990) An approach for time-dependent bone modeling and remodeling: theoretical development. J Orthop Res 8:651–661
Bergmann G, Graichen F, Rohlmann A (1993) Hip joint loading during walking and running, measured in two patients. J Biomech 26(8):969–990
Bougherara H, Klika V, Maršík F, Mařík I, Yahia L (2010) New predictive model for monitoring bone remodeling. J Biomed Mater Res Part A 95(1):9–24
Charopoulos I, Tournis S, Trovas G, Raptou P, Kaldrymides P, Skarandavos G, Katsalira K, Lyritis GP (2006) Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocr Metab 91(5):1748–1753
Cohen M Jr (2006) The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A 140(23):2646–2706
Collin-Osdoby P (2004) Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 95:1046–1057
Cowin SC, Hegedus DH (1976) Bone remodelling i: theory of adaptaive elasticity. J Elast 6:313–326
Currey JD (2004) Tensile yield in compact bone is determined by strain, post-yield behaviour by mineral content. J Biomech 37(4):549–556
Doblaré M, García J (2002) Anisotropic bone remodelling model based on a continuum damage-repair theory. J Biomech 35(1):1–17
Doblaré M, García J, Gómez M (2004) Modelling bone tissue fracture and healing: a review. Eng Fract Mech 71:1809–1840
Ettinger B, Pressman A, Sklarin P, Bauer DC, Cauley JA, Cummings SR (1998) Associations between low levels of serum estradiol, bone density, and fractures among elderly women: The study of osteoporotic fractures. J Clin Endocr Metab 83(7):2239–2243
Fang G, Ji B, Liu XS, Guo XE (2010) Quantification of trabecular bone microdamage using the virtual internal bond model and the individual trabeculae segmentation technique. Comput Method Biomech Biomed Eng 13(5):605–615
Frost HM (1963) Bone remodelling dynamics. C C Thomas, Springfield
Frost HM (2000) The utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab 18:305–316
Fukunaga T, Kurata K, Matsuda J, Higaki H (2008) Effects of strain magnitude on mechanical responses of three-dimensional gel-embedded osteocytes studied with a novel 10-well elastic chamber. J Biomech Sci Eng 3(1):13–24
Fyhrie DP, Schaffler MB (1995) The adaptation of bone apparent density to applied load. J Biomech 28:135–146
García-Aznar JM, Rueberg T, Doblaré M (2005) A bone remodelling model coupling microdamage growth and repair by 3D BMU-activity. Biomech Model Mechanobiol 4:147–167
Goemaere S, Van Laere M, De Neve P, Kaufman J (1994) Bone mineral status in paraplegic patients who do or do not perform standing. Osteoporosis Int 4(3):138–143
Gong J, Arnold J, Cohn S (1964) Composition of trabecular and cortical bone. Anat Rec 149(3):325– 331
Hazelwood SJ, Martin RB, Rashid MM, Rodrigo JJ (2001) A mechanistic model for internal bone remodeling exhibits different dynamic responses in disuse and overload. J Biomech 34:299–308
Hazenberg JG, Taylor D, Lee TC (2007) The role of osteocytes and bone microstructure in preventing osteoporotic fractures. Osteoporosis Int 18:1–8
Hernandez C (2001) Simulation of bone remodeling during the development and treatment of osteoporosis. Phd thesis, Stanford University, Stanford
Hernández-Gil I, Gracia M, Pingarrón M, Jerez L (2006) Physiological bases of bone regeneration ii. the remodeling process. Med Oral Patol Oral Cir Bucal 11:E151–215
Hill PA (1998) Bone remodelling. Brit J Orthod 25:101–107
Hjelmstad KD (2005) Fundamentals of structural mechanics. Springer, Berlin
Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD (2000) Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405:704–706
Ingber DE (2008) Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Bio 97: 163179
Jacobs CR (1994) Numerical simulation of bone adaptation to mechanical loading. Phd thesis, Stanford University, Stanford
Jacobs CR, Simo JC, Beaupré GS, Carter DR (1997) Adaptive bone remodeling incorporating simultaneous density and anisotropy considerations. J Biomech 30(6):603–613
Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T (1999) Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol 163(1):434–442
Kim C, You L, Yellowley C, Jacobs C (2006) Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through rankl and opg signaling. Bone 39(5):1043–1047
Klika V (2010) Comparison of the effects of possible mechanical stimuli on the rate of biochemical reactions. J Phys Chem B 114(32):10,567–10,572
Klika V, Maršík F (2009) Coupling effect between mechanical loading and chemical reactions. J Phys Chem B 113:14,689–14,697
Klika V, Maršík F (2010) A thermodynamic model of bone remodelling: The influence of dynamic loading together with biochemical control. J Musculoskelet Neuron Interact 10(3):220–230
Klika V, Maršík F (2011a) Biomechanics, vol 1, INTECH, Vienna, chap Feasible simulation of diseases related to bone remodelling and of their treatment. ISBN 978-953-307-312-5, [online] http://www.sciyo.com
Klika V, Maršík F (2011b) Feasible predictions of bone remodelling using modelling techniques. Locomot Appar 1+2:26–41, [online] http://www.pojivo.cz
Klika V, Maršík F, Mařík I (2010) Dynamic Modelling, INTECH, Vienna, chap Influencing the Effect of Treatment of Disease Related to Bone Remodelling by Dynamic Loading. ISBN 978-953-7619-68-8, [online] http://www.sciyo.com
Klika V, Grmela M (2013) Coupling between chemical kinetics and mechanics that is both nonlinear and compatible with thermodynamics. Phys rev E 87(1–1):012,141–012,141
Kobayashi Y, Udagawa N, Takahashi N (2009) Action of rankl and opg for osteoclastogenesis. Crit Rev Eukaryot Gene Expr 19(1):61
Komarova SV, Smith RJ, Dixon SJ, Sims SM, Wahl LM (2003) Mathematical model predicts a critical role for osteoclast autocrine regulation in the control of bone remodeling. Bone 33(2):206–215
Kroll MH (2000) Parathyroid hormone temporal effects on bone formation and resorption. Bull Math Biol 61(1):163–188
Kurata K, Heino TJ, Higaki H, Vaananen HK (2006) Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J Bone Miner Res 21(4):616–625
Lai Y, Qin L, Hung V, Chan K (2005) Regional differences in cortical bone mineral density in the weight-bearing long bone shafta pqct study. Bone 36(3):465–471
Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ (2004) Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J Theor Biol 229(3):293–309
Manolagas SC (1999) Cell number versus cell vigor-what really matters to a regeneration skeleton? Endocrinology 140(10):4377–4381
Martin RB (1995) A mathematical model for fatigue damage repair and stress fracture in osteonal bone. J Orthop Res 13:309–316
Martin RB (2007) Targeted bone remodeling involves BMU steering as well as activation. Bone 40(6):1574–1580
Martin T (2004) Paracrine regulation of osteoclast formation and activity: Milestones in discovery. J Musculoskelet Neuron Interact 4:243–253
Martínez-Reina J, García-Aznar JM, Dominguez J, Doblaré M (2008) On the role of bone damage in calcium homeostasis. J Theor Biol 254:704–712
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng J, Bonewald L, Kodama T, Wutz A, Wagner E, et al. (2011) Evidence for osteocyte regulation of bone homeostasis through rankl expression. Nat med 11:1231-1234
van Oers RF, Ruimerman R, Tanck E, Hilbers PA, Huiskes R (2008) A unified theory for osteonal and hemiosteonal remodeling. Bone 42(2):250–259
Parfitt AM (2002) Life history of osteocytes: relationship to bone age, bone remodeling and bone fragility. J Musculoskelet Neuron Interact 2(6):499–500
Pattin CA, Caler WE, Carter DR (1996) Cyclic mechanical property degradation during fatigue loading of cortical bone. J Biomech 29(1):69–79
Pivonka P, Zimak J, Smith D, Gardiner B, Dunstan C, Sims N, John Martin T, Mundy G (2008) Model structure and control of bone remodeling: a theoretical study. Bone 43(2):249–263
Pivonka P, Zimak J, Smith D, Gardiner B, Dunstan C, Sims N, John Martin T, Mundy G (2010) Theoretical investigation of the role of the rank-rankl-opg system in bone remodeling. J Theor Biol 262(2):306– 316
Prendergast PJ, Taylor D (1994) Prediction of bone adaptation using damage accumulation. J Biomech 27(8):1067–1076
Proff P, Römer P (2009) The molecular mechanism behind bone remodeling: a review. Clin Oral Invest 13:355–362
Qu C, Qin QH, Kang Y (2006) A hypothetical mechanism of bone remodeling and modeling under electromagnetic loads. Biomaterials 27:4050–4057
Ramtani S, Zidi M (2001) A theoretical model of the effect of continuum damage on a bone adaptation model. J Biomech 34(4):471–479
Rattanakul C, Lenbury Y, Krishnamara N, Wollkind DJ (2003) Modeling of bone formation and resorption mediated by parathyroid hormone: response to estrogen/PTH therapy. Biosystems 70:55–72
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498
Rodan G (1998) Bone homeostasis. Proc Natl Acad Sci USA 95(23):13,361–13,362
Rodan GA, Martin TJ (1981) Role of osteoblasts in hormonal control of bone resorption- a hypothesis. Calcif Tissue Int 33(4):349–351
Roux W (1881) Der zuchtende Kampf der teile, oder die Teilauslee im Organismus (Theorie der funktionellen anpassung). Wukgekn Ebgeknabb, Leipzig
Rubin J, Murphy T, Nanes MS, Fan X (2000) Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol-Cell Ph 278:C1126–C1132
Ryser M, Nigam N, Komarova S (2008) Mathematical modeling of spatio-temporal dynamics of a single bone multicellular unit. J Bone Miner Res 24(5):860–870
Sikavitsas VI, Temenoff JS, Mikos AG (2001) Biomaterials and bone mechanotransduction. Biomaterials 22:2581–2593
Skerry TM (1998) Methods in bone biology, 1st edn, chap 6, Chapman & Hall, London, UK, pp 149–176. ISBN 0 412 75770 2
Stacey E, Korkia P, Hukkanen M, Polak J, Rutherford O (1998) Decreased nitric oxide levels and bone turnover in amenorrheic athletes with spinal osteopenia. J Clin Endocr Metab 83(9):3056–3061
Tan S, de Vries T, Kuijpers-Jagtman A, Semeins C, Everts V, Klein-Nulend J (2007) Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone 41(5):745–751
Tudor-Locke C, Bassett J (2004) How many steps/day are enough?: Preliminary pedometer indices for public health. Sports Med 34(1):1–8
Vaira S, Alhawagri M, Anwisye I, Kitaura H, Faccio R, Novack DV (2008) RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest 118(6):2088–2097
Virtama P, Telkkä A (1962) Cortical thickness as an estimate of mineral content of human humerus and femur. Brit J Radiol 35(417):632–633
Wang H, Ji B, Liu XS, Guo XE, Huang Y, Hwang KC (2012) Analysis of microstructural and mechanical alterations of trabecular bone in a simulated three-dimensional remodeling process. J biomech 45:2417-2425
Wang H, Ji B, Liu X, Oers R, Guo X, Huang Y, Hwang KC (2013) Osteocyte-viability-based simulations of trabecular bone loss and recovery in disuse and reloading. Biomech Model Mechanobiol, pp 1–14. doi:10.1007/s10237-013-0492-1
Whalen RT, Carter DR, Steele CR (1988) Influence of physical activity on the regulation of bone density. J Biomech 21(10):825–837
Wimalawansa SJ (2007) Rationale for using nitric oxide donor therapy for prevention of bone loss and treatment of osteoporosis in humans. Ann NY Acad Sci 1117:283–297
Wolff J (1892) Das Gesetz der transformation der knochen. Hirchwild, Berlin
Acknowledgments
This research was supported by the Instituto Aragones de Ciencias de la Salud through the research project (PIPAMER10/015) and the Spanish Ministry of Science and Technology through the Research Project DPI2011-22413. Further support was received from the CENTEM project, reg. no. CZ.1.05/2.1.00/03.0088, that is partially supported by the ERDF within the OP RDI Programme of the Ministry of Education, Youth and Sports and from the institutional support RVO:61388998.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A: Relation between daily strain history \(\xi \) and the mechanical parameter \(\mathcal {D}_{\alpha }\)
The mechanical stimulus used in the biochemical model was
where \(d^{(1)}\) is the rate of volume variation and \(l_{v\alpha }\) are given in Table 8. In the mechano-biochemical model presented here, we generalise this relation by including influence of shear, see (6) and (7). The relation between the newly used form of mechanical stimulation \(\xi \) and the previous one \(\mathcal {D}_{\alpha }\) is the following:
where the value of \(A\) was determined from the reference healthy states, see the end of Sect. 2.2. The values of \(\mathcal {D}_{\alpha ,ref}\) are calculated from (23) where the value of \(d^{(1)}\) corresponds to the maximal strain rate found in a healthy mechanical stimulation, see Sect. 2.2 and the original model formulation (Klika and Maršík 2010):
Appendix B: Biochemical model
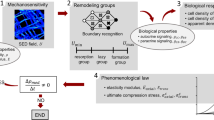
The model presented here is an updated version of (Klika and Maršík 2010; Klika et al. 2010; Klika and Maršík 2011a, b). The core of the biochemical part of the presented model is characterised by the following interactions:
where \(MNOC\) are multi-nucleated osteoclasts being formed by fusion from their precursors \(MCELL\) when appropriate receptor-ligand (cell-to-cell) interaction takes place (\(RR\) representing RANKL-RANK interaction mediated by the RANKL-RANK-OPG chain), \(Old\_B\) denotes old bone that might be resorbed, the released local factors from bone are denoted by \(LF\) which activate \(osteoprogenitor\) cells that proliferate and differentiate into osteoblasts \(OB\), which secrete \(osteoid\) (non-mineralised bone - organic part of bone tissue). The ossification of \(osteoid\) into new bone tissue \(New\_B\) happens after addition of appropriate substances, \(N_{14}\), takes place. \(N_4, N_7, N_{10}, N_{13}, N_{16}\) are waste products. For a more detailed description see (Klika and Maršík 2010).
The RANKL-RANK-OPG pathway is considered as follows:
Finally, the effect of estradiol, PTH, and NO is translated through this pathway:
where \(OPG_{\mathrm{prod}},~RANKL_{\mathrm{prod}}\) represent the group of cells that expresses OPG, RANKL and a mixture of substances needed for osteoprotegerin, RANKL production is noted as \(Subst\), respectively and \(Remaining\_prod\) represents inactivated RANKL.
The dimensionless system of differential equations describing the kinetics of interactions of the core of the biochemical part of the presented model model (24) together with the influence of mechanical stimulus \(\mathcal {D}_{\alpha }(\xi )\) (for the relationship between \(\mathcal {D}_{\alpha }\) and \(\xi \) see appendix 5) is provided here
where \(\beta _{i}\) is the sum of normalised initial molar concentrations of relevant substances, \(\delta _{\alpha }\) relates to the interaction rate, \(D_{\alpha }(\xi )\) describes the influence of dynamic loading on reactions, \(n_{i}\) is the normalised concentration of the \(i\)-th substance, \(\mathcal {J}_3\) is a normalised outflow of \(MNOC\) (i.e., \(MNOC\) apoptosis) and \(\mathcal {J}_{New\_B}\) is the normalised outflow of new bone \(New\_B\) into old bone \(Old\_B\).
By solving these kinetic equations, the time evolution of \([\mathrm{MCELL}], [\mathrm{Old\_B}], [\mathrm{OB}], [\mathrm{Osteoid}]\), and \([\mathrm{New\_B}]\) concentrations can be obtained: the relation between concentrations and their dimensionless form is:
where \(k_{+2}=6 \cdot 10^7 \frac{l}{mol\cdot s}\) and \([\mathrm{Bo}]=2.2 \frac{10^{-4}}{N_A} \frac{mol}{\mu m^3}\) with \(N_A\) being the Avogardo’s number. In this model, it is assumed that the density of bone tissue is proportional to the number of osteocytes in a given volume, or that the molar concentration of osteocytes in tissue is constant; the amount of bone tissue in a given volume then determines the local bone density. Thus the quantity \([\mathrm{Old\_B}]\) is (by definition) equal to the concentration of osteocytes \([\mathrm{OCy}]\) which, with respect to above mentioned assumption, gives us the concentration of bone tissue \(Old\_B\):
which directly relates the maximal normalised \(n_{Old\_B}\) to maximal apparent bone tissue density \([\mathrm{Old\_B}]\) found in the human femur. The kinetics of other interactions is derived from the Law of Mass Action or can be found in the referred literature. Here, to provide a stand-alone model, the remaining differential equations of the biochemical model describing the considered kinetics of RANKL-RANK-OPG pathway are recapitulated.
RANKL-RANK-OPG pathway The law of mass action gives for the RANKL-RANK-OPG pathway (25) the following system of differential equations where we denote the dimensionless concentration of RANKL and OPG as \(n_{RKL}\) and \(n_{OPG}\), respectively:
with \(n_{RR}(t)=\beta _{\mathrm{RR}}^{\mathrm{RRO}}-n_{RKL}(t)+n_{OPG}(t)\) and parameter values are given in Appendix 8. The amount of RANKL-RANK bonds (\(RR\)) mediate the outcome effect of the whole RANKL-RANK-OPG pathway on bone remodelling. The amount of \(RR\) bonds is included in the \(\beta _1\) parameter. The differential equations are in dimensionless form and to provide prediction of changes in real serum levels they need to be compared with the physiological value of RANKL that was used for obtaining the dimensionless form. In total, the initial conditions are given by relationships
and standard serum levels in humans were estimated to be \([\mathrm{RKL}]_{st}=0.84\frac{pmol}{l}=46.2\frac{pg}{ml}\) and \([\mathrm{OPG}]_{st}=1.8\frac{pmol}{l}=36\frac{pg}{ml}\). The normalised standard OPG concentration \(n_{OPG,st}\) is determined from molar masses of RANKL and OPG, see (Klika et al. 2010). Finally, the interconnection with the core model is
giving \(\beta _1=0.6\) for standard serum values of RANKL and OPG. For more details see (Klika et al. 2010).
As was mentioned, many of the factors having a significant influence on RANKL-RANK-OPG signalling pathway, and thus on bone quality, are translated through this same pathway. Below we will add impacts of PTH, estradiol and nitric oxide. Here, we model their impact by modifying the initial values of RANKL and OPG in this RANKL-RANK-OPG model as predicted from the following sub-models. The final initial conditions for RANKL and OPG are determined by the sum of all predicted deflections from their standard values, namely
where the terms in brackets denote differences from standard initial values caused by the control mechanism noted in the upper indices.
Further, as each following sub-model has its own normalisation there are two scaling factors present in each one: (1) scaling of used in-vitro data by \(C_{in-vitro}\) (it is unclear if this scaling finds an equivalent amount, in terms of its effects on bone remodelling, of in-vivo serum levels to that of used in-vitro ones but it seems to capture their impact on bone remodelling as reported in literature), (2) scaling to match the different normalisation used— matching is such that standard value of a given factor are met in both normalisations by \(C_{norm}\), see below.
PTH The impact of PTH on RANKL-RANK-OPG pathway (28) can be described as follows:
which affects the amount of RANKL available for making receptor-ligand bonds. This equation for normalised in-vitro concentration \(\tilde{n}_{PTH}\) was fitted with in-vitro dose-dependent data (no other available). The rescaling of in-vitro data is included in \({\tilde{n}_{PTH}}=C_{in-vitro}^{PTH} n_{PTH}\), \(C_{in-vitro}^{PTH}=0.296\) and thus the initial condition needed for solving the above differential equation is
where \([\mathrm{PTH}]\) represents serum levels of PTH, typically in \(\frac{pg}{ml}\). The concentration of RANKL after action of PTH is (\(n_{PTH}(\tau )\) being the scaled solution of the above differential equation):
where \(n_{RKL_0}^{PTH}\) represents the initial concentration of RANKL before interacting with PTH according to (28) and \(C_{norm}^{PTH}=1.0\) is the second scaling to match the different normalisations (see above). The amount of available RANKL for forming bonds with its receptor RANK is denoted as \(n_{RKL_0}^{RRO,PTH}\) and is used as an input into the RANKL-RANK-OPG model taking into account the influence of PTH on RANKL concentration, see (31)–(32). Parameter values can be found again in Appendix 8.
Estradiol The idea behind adding an impact of estradiol on RANKL-RANK-OPG pathway (27) is the same as in PTH, only that estradiol affects OPG instead of RANKL (over-tilde has the same meaning as in PTH—rescaled values of estradiol):
and the initial condition is obtained from serum levels analogously to that one in PTH, Eq. (33), with \(C_{in-vitro}^{estr}=1.19\). The concentration of OPG after action of estradiol is
with \(C_{norm}^{estr}=1.04\). Again the amount of available OPG for blocking bond formation is denoted as \(n_{OPG_0}^{RRO,estr}\) and is used as an input into the RANKL-RANK-OPG model taking into account the influence of estradiol on OPG concentration, see (31)-(32). Parameter values can be found again in Appendix 8.
Nitric oxide Similarly, the influence of nitric oxide on RANKL-RANK-OPG pathway (29)–(30) can be included. It affects both RANKL and OPG concentrations. It was assumed that the effects of NO on OPG and RANKL can be separated (Klika and Maršík 2011a) leading to the same differential equations as in previous cases for estradiol and PTH. Due to unavailability of in-vivo data that could quantify NO effects on bone quality we used studies where the amount of NO was regulated by NO donor intake in milligrams per day (typically nitroglycerin). A non-linear scaling in the input data was required to obtain a fit with observed outcome effects on BMD. It is unclear if this scaling finds an equivalent amount (in terms of its effects on bone remodelling) of in-vivo NO serum levels to that of donor but it seems to capture the impact of NO on bone remodelling. Concretely, the scaling transforms were logarithmic \({\tilde{n}_{NO,OPG}}=C_{in-vitro}^{NO,OPG}\left( 1+\ln \left( [\mathrm{NO^{OPG}}]/[\mathrm{NOdonor}]_{stand}\right) \right) \) and \({\tilde{n}_{NO^{RKL}}}=C_{in-vitro}^{NO,RKL}\left( 1+\ln \left( [\mathrm{NO^{RKL}}]/[\mathrm{NOdonor}]_{stand}\right) \right) \) with normalisation constants \(C_{in-vitro}^{NO,OPG}=1.01\), \(C_{in-vitro}^{NO,RKL}=0.036\) and with \([\mathrm{NOdonor}]_{stand}=0.044\frac{mg}{kg\times day}\) being the standard or reference amount of NO donor that does not cause any change in BMD (Wimalawansa 2007).
Finally, the differences from standard values of OPG and RANKL caused by NO are
where the two normalisation constants are \(C_{norm}^{NO,RKL}=1.02\), \(C_{norm}^{NO,OPG}=2.16\). Again the amount of available OPG for blocking bond formation is denoted as \(n_{OPG_0}^{RRO,NO},~n_{RKL_0}^{RRO,NO}\) and is used as an input into the RANKL-RANK-OPG model taking into account the influence of nitric oxide on this essential control pathway, see (31)–(32). Parameter values can be found in Appendix 8.
Implementation of the whole model in Abaqus as a user subroutine is enclosed in the supplementary material.
Appendix C: Stationary solution
The biochemical model, see Sect. 6, has exactly one positive stationary solution:
which for the considered parameter values fulfil all the assumptions of Poincare-Ljapunov theorem and thus the stationary solution is an asymptotically stable fixed point.
Appendix D: List of parameter values
Rights and permissions
About this article
Cite this article
Klika, V., Pérez, M.A., García-Aznar, J.M. et al. A coupled mechano-biochemical model for bone adaptation. J. Math. Biol. 69, 1383–1429 (2014). https://doi.org/10.1007/s00285-013-0736-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-013-0736-9


 :
: and its j-
and its j-