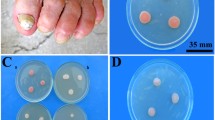
Abstract
Pigments produced by micro-organisms could contribute to their pathogenesis and resistance. The investigation into the red pigment of R. mucilaginosa and its ability to survive and resist has not yet been explored. This study aimed to investigate the survival and resistance of the R. mucilaginosa CQMU1 strain following inhibition of pigment production by naftifine and its underlying mechanism. The red-pigmented Rhodotorula mucilaginosa CQMU1 yeast was isolated from an infected toenail of a patient with onychomycosis. Cultivation of R. mucilaginosa in liquid and solid medium showed the effect of naftifine after treatment. Then, analysis of phagocytosis and tolerance to heat or chemicals of R. mucilaginosa was used to evaluate the survival and resistance of yeast to different treatments. Naftifine reversibly inhibited the pigmentation of R. mucilaginosa CQMU1 in solid and liquid media. Depigmented R. mucilaginosa CQMU1 showed increased susceptibility toward murine macrophage cells RAW264.7 and reduced resistance toward different types of chemicals, such as 1.5-M NaCl and 0.5% Congo red. Inhibition of pigment production by naftifine affected the survival and growth of R. mucilaginosa and its resistance to heat and certain chemicals. The results obtained could further elucidate the target of new mycosis treatment.





Similar content being viewed by others
Data Availability
The data of this study are presented in the article text and tables. Additional details are available by contacting the corresponding author upon reasonable request. Some data are provided in full in the results sections of this paper and as supplementary data.
Code Availability
Not applicable.
References
Yang Q, Li Y, Apaliya MT, Zheng X, Serwah BNA, Zhang X, Zhang H (2018) The response of Rhodotorula mucilaginosa to patulin based on lysine crotonylation. Front Microbiol 9:2025. https://doi.org/10.3389/fmicb.2018.02025
Miceli MH, Díaz JA, Lee SA (2011) Emerging opportunistic yeast infections. Lancet Infect Dis 11(2):142–151. https://doi.org/10.1016/S1473-3099(10)70218-8
Ioannou P, Vamvoukaki R, Samonis G (2019) Rhodotorula species infections in humans: a systematic review. Mycoses 62(2):90–100. https://doi.org/10.1111/myc.12856
Jarros IC, Barros ILE, Prado A, Corrêa JL, Malacrida AM, Negri M, Svidzinski TIE (2022) Rhodotorula sp. and Trichosporon sp. are more virulent after a mixed biofilm. Mycopathologia 187(1):85–93. https://doi.org/10.1007/s11046-021-00606-5
Idris NFB, Huang G, Jia Q, Yuan L, Li Y, Tu Z (2019) Mixed infection of toe nail caused by Trichosporon asahii and Rhodotorula mucilaginosa. Mycopathologia 185:373–376. https://doi.org/10.1007/s11046-019-00406-y
Merhan O (2017) Biochemistry and antioxidant properties of carotenoids. Carotenoids 5:51. https://doi.org/10.5772/67592
Milani A, Basirnejad M, Shahbazi S, Bolhassani A (2017) Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 174(11):1290–1324. https://doi.org/10.1111/bph.13625
Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V (2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202(2):209–215. https://doi.org/10.1084/jem.20050846
Chen F, Di H, Wang Y, Cao Q, Xu B, Zhang X, Yang N, Liu G, Yang CG, Xu Y, Jiang H, Lian F, Zhang N, Li J, Lan L (2016) Small-molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nat Chem Biol 12(3):174–179. https://doi.org/10.1038/nchembio.2003
Moliné M, Flores MR, Libkind D et al (2010) Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: the role of torularhodin. Photochem Photobiol Sci 9:1145–1151. https://doi.org/10.1039/c0pp00009d
Zelver N, Hamilton M, Pitts B, Goeres D, Walker D, Sturman P, Heersink J (1999) Measuring antimicrobial effects on biofilm bacteria: from laboratory to field. Methods Enzymol 310:608–628. https://doi.org/10.1016/s0076-6879(99)10047-8
Tuon FF, Costa SF (2008) Rhodotorula infection. A systematic review of 128 cases from literature. Rev Iberoam Micol 25:135–140. https://doi.org/10.1016/s1130-1406(08)70032-9
Davoli P, Mierau V, Weber RWS (2004) Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl Microbiol Biotechnol 40:392–397. https://doi.org/10.1023/B:ABIM.0000033917.57177.f2
Moț AC, Pârvu M, Pârvu AE, Roşca-Casian O, Dina NE, Leopold N, Silaghi-Dumitrescu R, Mircea C (2017) Reversible naftifine-induced carotenoid depigmentation in Rhodotorula mucilaginosa (A. Jörg.) FC Harrison causing onychomycosis. Sci Rep 7(1):1–12. https://doi.org/10.1038/s41598-017-11600-7
Li B, Ni S, Chen F, Mao F, Wei H, Liu Y et al (2018) Discovery of potent benzocycloalkane derived diapophytoene desaturase inhibitors with an enhanced safety profile for the treatment of MRSA. VISA, and LRSA infections. ACS Infect Dis 4:208–217. https://doi.org/10.1016/j.ejmech.2017.12.090
Huang G, Idris NFB, Li Y, Wang Y, Tu Z (2020) Naftifine inhibits pigmentation through down-regulation on expression of phytoene desaturase gene CAR1 in Rhodotorula mucilaginosa. Afr J Microbiol Res 14(5):166–174. https://doi.org/10.5897/AJMR2020.9316
Padyana AK, Gross S, Jin L, Cianchetta G, Narayanaswamy R, Wang F, Wang R, Fang C, Lv X, Biller SA, Dang L, Mahoney CE, Nagaraja N, Pirman D, Sui Z, Popovici-Muller J, Smolen GA (2019) Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nat Commun 10:97. https://doi.org/10.1038/s41467-018-07928-x
Nosanchuk JD, Casadevall A (2006) Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother 50:3519–3528. https://doi.org/10.1128/AAC.00545-06
Jahn B et al (1997) Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun 12:5110–5117. https://doi.org/10.1128/iai.65.12.5110-5117.1997
Pihet M et al (2009) Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol 9:177. https://doi.org/10.1186/1471-2180-9-177
Koselny K, Mutlu N, Minard AY, Kumar A, Krysan DJ, Wellington M (2018) A genome-wide screen of deletion mutants in the filamentous Saccharomyces cerevisiae background identifies ergosterol as a direct trigger of macrophage pyroptosis. MBio 9(4):e01204-e1218. https://doi.org/10.1128/mBio.01204-18
Schroeder WA, Johnson EA (1993) Antioxidant role of carotenoids in Phaffia rhodozyma. J Ind Microbiol Biotechnol 139:907–912. https://doi.org/10.1099/00221287-139-5-907
Kejžar A, Gobec S, Plemenitaš A, Lenassi M (2013) Melanin is crucial for growth of the black yeast Hortaea werneckii in its natural hypersaline environment. Fungal Biol 117(5):368–379. https://doi.org/10.1016/j.funbio.2013.03.006
Leber R, Landl K, Zinser E, Ahorn H, Spok A, Kohlwein SD, Turnowsky F, Daum G (1998) Dual localization of squalene epoxidase, SQLE protein, in yeast reflects a relationship between the endoplasmic reticulum and lipid particles. Mol Biol Cell 9:375–386. https://doi.org/10.1091/mbc.9.2.375
Wentzinger LF, Bach TJ, Hartmann MA (2002) Inhibition of squalene synthase and squalene epoxidase in tobacco cells triggers an up-regulation of 3-hydroxy-3-methylglutaryl coenzyme a reductase. Plant Physiol 130:334–346. https://doi.org/10.1104/pp.004655
Gill S, Stevenson J, Kristiana I, Brown AJ (2011) Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab 13(3):260–273. https://doi.org/10.1016/j.cmet.2011.01.015
Germann M, Gallo C, Donahue T, Shirzadi R et al (2005) Characterizing sterol defect suppressors uncovers a novel transcriptional signaling pathway regulating zymosterol biosynthesis. J Biol Chem 280:35904–35913. https://doi.org/10.1074/jbc.M504978200
Rosas AL, Casadevall A (1997) Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett 153:265–272
Paolo WF, Dadachova E, Mandal P et al (2006) Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol 6:55. https://doi.org/10.1186/1471-2180-6-55
Agustinho DP, and Nosanchuk J (2017). Functions of Fungal Melanins. In book Reference Module in Life Science. https://doi.org/10.1016/B978-0-12-809633-8.12091-6.
Acknowledgements
We are grateful to The first Affiliated Hospital of Chongqing Medical University for providing the clinical isolates and laboratory equipment.
Funding
This research was funded by Chongqing Research Program of Basic Research and Frontier Technology [Grant No. cstc2021jcyj-msxmX0158], and Scientific and Technological Research Program of Chongqing Municipal Education Commission [Grant No. KJQN202113201]. The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
NFBI performed the experiments (lead), analyzed the data (lead), and wrote the original draft of the manuscript (lead). QJ (supporting) and HL (supporting) conducted sampling and sample identification, and YG (supporting) and WY provided assistance in the purchase of chemicals (lead) and culture media preparation (supporting). H provided assistance with the generation of graphical data (supporting) and manuscript editing. TZ designed the study (lead) and supervised the work (lead) and was responsible for conceptualization (lead), data curation (lead), funding acquisition (lead), and methodology formulation (lead). All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no conflicts of interest.
Ethical Approval
The Ethics Committee of Chongqing Medical University has approved this study as reference number 2020211 and the approval was given on 10th October 2020.
Informed Consent
Informed consent was obtained from all individual participants, authors, and patients, included in the study.
Consent to Participations
The patient of Chongqing Medical University has given the consent to participate in this study as approved by the Ethics Committee of Chongqing Medical University given on 10th October 2020 with reference number 2020011.
Consent for Publications
The Ethics Committee of Chongqing Medical University has approved this study as reference number 2020211 and the data obtained can be published in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Idris, N.F.B., Jia, Q., Lu, H. et al. Reduced Survival and Resistance of Rhodotorula mucilaginosa Following Inhibition of Pigment Production by Naftifine. Curr Microbiol 80, 285 (2023). https://doi.org/10.1007/s00284-023-03388-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03388-9