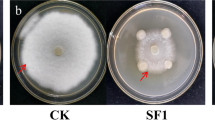
Abstract
Streptomyces strains were isolated from rhizosphere soil and evaluated for in vitro plant growth and antagonistic potential against Ralstonia solanacearum. Based on their in vitro screening, seven Streptomyces were evaluated for plant growth promotion (PGP) and biocontrol efficacy by in-planta and pot culture study. In the in-planta study, Streptomyces-treated eggplant seeds showed better germination percentage, plant growth, and disease occurrence against R. solanacearum than the control treatment. Hence, all seven Streptomyces cultures were developed as a bioformulation by farmyard manure and used for pot culture study. The highest plant growth, weight, and total chlorophyll content were observed in UP1A-1-treated eggplant followed by UP1A-4, UT4A-49, and UT6A-57. Similarly, the maximum biocontrol efficacy was observed in UP1A-1-treated eggplants against bacterial wilt. The biocontrol potential of Streptomyces is also confirmed through metabolic responses by assessing the activities of the defense-related enzymes peroxidase (POX), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) and as well as the levels of total phenol. Treatment with UP1A-1/ UT4A-49 and challenge with R. solanacearum led to maximum changes in the activities of POX, PPO, and PAL and the levels of total phenol in the eggplants at different time intervals. Alterations in enzymes of UP1A-1 treatment were related to early defense responses in eggplant. Therefore, the treatment with UP1A-1 significantly delayed the establishment of bacterial wilt in eggplant. Altogether, the present study suggested that the treatment of Streptomyces maritimus UP1A-1 fortified farmyard manure has improved the plant growth and stronger disease control against R. solanacearum on eggplant.




Similar content being viewed by others
References
Rajmohan KS, Chandrasekaran R, Varjani S (2020) A review on occurrence of pesticides in environment and current technologies for their remediation and management. Ind J Microbiol 60:125–138
Panth M, Hassler SC, Baysal-Gurel F (2020) Methods for management of soilborne diseases in crop production. Agriculture 10:16
Sowndarya J, Rubini D, Sinsinwar S, Senthilkumar M, Nithyanand P, Vadivel V (2020) Gallic acid an agricultural byproduct modulates the biofilm matrix exopolysaccharides of the phytopathogen Ralstonia solanacearum. Curr Microbiol 77:3339–3354
Manda RR, Addanki VA, Srivastava S (2020) Bacterial wilt of solanaceous crops. Int J Chem Studies 8:1048–1057
Elsayed TR, Jacquiod S, Nour EH, Sørensen SJ, Smalla K (2020) Biocontrol of bacterial wilt disease through complex interaction between tomato plant, antagonists, the indigenous rhizosphere microbiota, and Ralstonia solanacearum. Front Microbiol 10:2835
Cheema MT, Ponomareva LV, Liu T, Randal Voss S, Thorson JS, Shaaban KA, Sajid I (2021) Taxonomic and metabolomics profiling of actinobacteria strains from Himalayan collection sites in Pakistan. Curr Microbiol 78:3044–3057
Laassami A, Yekkour A, Meklat A, Djemouai N, Zitouni A, Mokrane S, Lecomte P, Rey P, Berraf-Tebbal A (2020) Actinobacteria associated with vineyard soils of Algeria: classification, antifungal potential against grapevine trunk pathogens and plant growth-promoting features. Curr Microbiol 77:2831–2840
Dutta J, Thakur D (2020) Evaluation of antagonistic and plant growth promoting potential of Streptomyces sp. TT3 isolated from tea (Camellia sinensis) rhizosphere soil. Curr Microbiol 77:1829–1838
Minuto A, Spadaro D, Garibaldi A, Gullino ML (2006) Control of soilborne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization. Crop Prot 25:468–475
Zeng W, Wang D, Kirk W, Hao J (2012) Use of Coniothyrium minitans and other microorganisms for reducing Sclerotinia sclerotiorum. Biol Control 60:225–232
Gordon SA, Weber RP (1950) Colorimetric estimation of Indole Acetic Acid. pp 192–195
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology 17:362–370
Hu UP, Xu JG (2011) A simple double-layered chrome azurol S agar (SD-CASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. Afr J Microbiol Res 5:4321–4327
Cappuccino JG, Sherman N (2002) Microbiology. A laboratory manual. 6th edition. Pearson education inc. San Francisco, California. 215–224.
Cattelan AJ, Hartel PG, Fuhrmann JJ (1999) Origin of Bacteria. Soil Sci Soc Am J 63:1670–1680
Saadoun I, Muhana A (2008) Optimal production conditions, extraction, partial purification and characterization of inhibitory compound(s) produced by Streptomyces Ds-104 isolate against multi-drug resistant Candida albicans. Curr Trends Biotechnol 3:402–420
Manigundan K, Sakthivel K, Gautam RK, Kumar K, Anantharaj A, Velumurugan A, Singh PK, Dam Roy S (2016) Characterization of species from vegetable and spice rhizospheres of Andaman Islands for broad spectrum antagonism and plant growth promotion. J Environ Biol 37:341–347
Manigundan K, Jerrine J, Radhakrishnan M, Ayswarya S, Gopikrishnan V, Balamurugan A, Sakthivel K (2021) Anti-biofilm activity and biocontrol potential of Streptomyces cultures against Ralstonia solanacearum on tomato plants. Indian J Microbiol. https://doi.org/10.1007/s12088-021-00963-1
Ni Z, Kim ED, Chen ZJ (2009) Chlorophyll and starch assays. Protocol Exchange. https://doi.org/10.1038/nprot.2009.12
Zieslin N, Ben-Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flower. Plant Physiol Biochem 31:333–339
Salla TD, Ramos da Silva T, Astarita LV, Santarém ER (2014) Streptomyces rhizobacteria modulate the secondary metabolism of Eucalyptus plants. Plant Physiol Biochem 85:14–20
Gothwal RK, Nigam VK, Mohan MK, Sasmal D, Ghosh P (2007) Extraction of bulk DNA from Thar desert soils for optimization of PCR-DGGE based microbial community analysis. Electron J Biotechnol 10:400–408
Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D (2007) Genomics of actinobacteria : Tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev 71:495–548
Priyadharsini P, Dhanasekaran D (2015) Diversity of soil allelopathic actinobacteria in Tiruchirappalli district, Tamil Nadu, India. J Saudi Soc Agric Sci 14:54–60
Passari AK, Mishra VK, Gupta VK, Yadav MK, Saikia R, Singh BP (2015) In vitro and in vivo plant growth promoting activities and DNA fingerprinting of antagonistic endophytic actinomycetes associates with medicinal plants. PLoS ONE 10:0139468
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: Diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649–655
Abd-alla MH, Rasmey AM (2013) Indole-3-acetic acid (IAA) production by Streptomyces atrovirens isolated from rhizospheric soil in Egypt. J Biol Earth Sci 3:182–193
Anwar S, Ali B, Sajid I (2016) Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front Microbiol 7:1334
Jha CK, Patel B, Saraf M (2012) Stimulation of the growth of Jatropha curcas by the plant growth promoting bacterium Enterobacter cancerogenus MSA2. World J Microbiol Biotechnol 28:891–899
Thilagam R, Hemalatha N (2019) Plant growth promotion and chilli anthracnose disease suppression ability of rhizosphere soil actinobacteria. J Appl Microbiol 126:1835–1849
Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney RK (2016) Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz J Microbiol 47:85–95
Sturz AV, Nowak J (2000) Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl Soil Ecol 15:183–190
Lasudee K, Tokuyama S, Lumyong S, Pathom-Aree W (2018) Actinobacteria Associated with arbuscular mycorrhizal funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and Thai Jasmine Rice (Oryza sativa). Front Microbiol 9:1247
Kunova A, Bonaldi M, Saracchi M, Pizzatti C, Chen X, Cortesi P (2016) Selection of Streptomyces against soil borne fungal pathogens by a standardized dual culture assay and evaluation of their effects on seed germination and plant growth. BMC Microbiol 16:272
Kinkel LL, Schlatter DC, Bakker MG, Arenz BE (2012) Streptomyces competition and co-evolution in relation to plant disease suppression. Res Microbiol 163:490–499
Tan HM, Cao LX, He ZF, Su GJ, Lin B, Zhou SN (2006) Isolation of endophytic actinomycetes from different cultivars of tomato and their activities against Ralstonia solanacearum in vitro. World J Microbiol Biotechnol 22:1275–1280
Ling L, Han X, Li X, Zhang X, Wang H, Zhang L, Cao P, Wu Y, Wang X, Zhao J, Xiang W (2020) A Streptomyces Sp. NEAU-HV9: Isolation, identification, and potential as a biocontrol agent against Ralstonia solanacearum of tomato plants. Microorganisms 8:351
Singh N, Phukan T, Sharma PL, Kabyashree K, Barman A, Kumar R, Sonti RV, Genin S, Ray SK (2018) An innovative root inoculation method to study Ralstonia solanacearum pathogenicity in tomato seedlings. Phytopathology 108:436–442
Singh V, Mawar R, Lodha S (2012) Combined effects of biocontrol agents and soil amendments on soil microbial populations, plant growth and incidence of charcoal rot of cowpea and wilt of cumin. Phytopathol Mediterr 51:307–316
Garkoti A, Kumar V, Tripathi HS (2014) Control of wilt disease of lentil through biocontol agents and organic amendments in Tarai region of Uttarakhand, India. J Environ Biol 35:1067–1070
Schonfeld J, Gelsomino A, van Overbeek LS, Gorissen A, Smalla K, van Elsas JD (2003) Effects of compost addition and simulated solarisation on the fate of Ralstonia solanacearum biovar 2 and indigenous bacterial in soil. FEMS Microbiol Ecol 43:63–74
Yadessa GB, van Bruggen AHC, Ocho FL (2010) Effects of different soil amendments on bacterial wilt caused by Ralstonia solanacearum and on the yield of tomato. J Plant Pathol 92:439–450
Zheng X, Zhu Y, Wang J, Wang Z, Liu B (2019) Combined use of a microbial restoration substrate and avirulent Ralstonia solanacearum for the control of tomato bacterial wilt. Sci Rep 9:20091
Kim HJ, Lee EJ, Park SH, Lee H, Chung N (2014) Biological control of anthracnose (Colletotrichum gloeosporioides) in pepper and cherry tomato by Streptomyces sp. A1022. J Agric Sci 6:54–62
Kurth F, Mailander S, Bonn M, Feldhahn L, Herrmann S, Grobe I, Buscot F, Schrey SD, Tarkka MT (2014) Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol Plant-Microbe Interact 27:891–900
Mandal S, Mitra A (2007) Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol Mol Plant Path 71:201–209
Enebe MC, Babalola OO (2019) The impact of microbes in the orchestration of plants’ resistance to biotic stress: a disease management approach. Appl Microbiol Biotechnol 103:9–25
Abbasi S, Safaie N, Sadeghi A, Shamsbakhsh M (2019) Streptomyces strains induce resistance to Fusarium oxysporum f Sp Lycopersici race 3 in tomato through different molecular mechanisms. Front Microbiol 10:1505
Salla TD, Astarita LV, Santarem ER (2016) Defense responses in plants of Eucalyptus elicited by Streptomyces and challenged with Botrytis cinerea. Planta 243:1055–1070
Acknowledgements
Authors acknowledge the Sathyabama Institute of Science and Technology (SIST), Chennai, Tamil Nadu for the research facilities provided.
Funding
The authors thank the Department of Biotechnology, India (BT/PR10814/AAQ/3/669/2014) and National Centre for Polar and Ocean Research, Ministry of Earth Sciences (MoES-NCPOR/ R.No.NCPOR/2019/PACER-POP/BS-08 dated 05-07-2019), for their support in the form of research grant.
Author information
Authors and Affiliations
Contributions
MK carried out sample collection, lab experiments, data analysis, and writing first draft of the manuscript. JJ and RM gave design, supervision, and revision of the manuscript. AS and GV contributed in lab experiments and manuscript writing. BA contributed to work on particular pathogen R. solanacearum and to the experimentation. SK supervised the research work and revised the article. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaari, M., Joseph, J., Manikkam, R. et al. Biocontrol Streptomyces Induces Resistance to Bacterial Wilt by Increasing Defense-Related Enzyme Activity in Solanum melongena L. Curr Microbiol 79, 146 (2022). https://doi.org/10.1007/s00284-022-02832-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02832-6