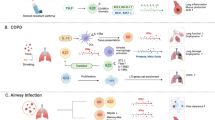
Abstract
The mucosal surface is in constant contact with foreign antigens and is regulated by unique mechanisms that are different from immune responses in the peripheral organs. For the last several decades, only adaptive immune cells such as helper T (Th) cells, Th1, Th2, or Th17 were targeted to study a wide variety of immune responses in the mucosal tissues. However, since their discovery, innate lymphoid cells (ILCs) have been attracting attention as a unique subset of immune cells that provide border defense with various functions and tissue specificity. ILCs are classified into different groups based on cell differentiation and functions. Group 3 innate lymphoid cells (ILC3s) are particularly in close proximity to mucosal surfaces and therefore have the opportunity to be exposed to a variety of bacteria including pathogenic bacteria. In recent years, studies have also provided much evidence that ILC3s contribute to disease pathogenesis as well as the defense of mucosal surfaces by rapidly responding to pathogens and coordinating other immune cells. As the counterpart of helper T cells, ILC3s together with other ILC subsets establish the immune balance between adaptive and innate immunity in protecting us from invasion or encounter with non-self-antigens for maintaining a complex homeostasis. In this review, we summarize recent advances in our understanding of ILCs, with a particular focus on the function of ILC3s in their involvement in bacterial infection and disease pathogenesis.

Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E (2013) Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 13:145–149
Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H (2018) Innate lymphoid cells: 10 years on. Cell 174:1054–1066
Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP (2008) Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29:958–970
Adachi S, Yoshida H, Kataoka H, Nishikawa S (1997) Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol 9:507–514
Seillet C, Luong K, Tellier J, Jacquelot N, Shen RD, Hickey P, Wimmer VC, Whitehead L, Rogers K, Smyth GK, Garnham AL, Ritchie ME, Belz GT (2020) The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat Immunol 21:168–177
Jarade A, Garcia Z, Marie S, Demera A, Prinz I, Bousso P, Di Santo JP, Serafini N (2022) Inflammation triggers ILC3 patrolling of the intestinal barrier. Nat Immunol 23:1317–1323
Gerold G, Zychlinsky A, de Diego JL (2007) What is the role of Toll-like receptors in bacterial infections? Semin Immunol 19:41–47
Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464:1371–1375
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289
Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14:275–281
Bishu S, Hou G, El Zaatari M, Bishu SR, Popke D, Zhang M, Grasberger H, Zou W, Stidham RW, Higgins PDR, Spence JR, Kamada N, Kao JY (2019) Citrobacter rodentium Induces Tissue-Resident Memory CD4+ T Cells. Infect Immun 87(7):e00295-19. https://doi.org/10.1128/IAI.00295-19
Ahlfors H, Morrison PJ, Duarte JH, Li Y, Biro J, Tolaini M, Di Meglio P, Potocnik AJ, Stockinger B (2014) IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J Immunol 193:4602–4613
Peeters PM, Wouters EF, Reynaert NL (2015) Immune homeostasis in epithelial cells: evidence and role of inflammasome signaling reviewed. J Immunol Res 2015:828264
Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G (2011) RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol 12:320–326
Jakob MO, Murugan S, Klose CSN (2020) Neuro-immune circuits regulate immune responses in tissues and organ homeostasis. Front Immunol 11:308
Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L (2012) The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36:92–104
Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, Eberl G, Baldassano RN, Laufer TM, Elson CO, Sonnenberg GF (2015) Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science 348:1031–1035
Melo-Gonzalez F, Kammoun H, Evren E, Dutton EE, Papadopoulou M, Bradford BM, Tanes C, Fardus-Reid F, Swann JR, Bittinger K, Mabbott NA, Vallance BA, Willinger T, Withers DR, Hepworth MR (2019) Antigen-presenting ILC3 regulate T cell-dependent IgA responses to colonic mucosal bacteria. J Exp Med 216:728–742
von Burg N, Chappaz S, Baerenwaldt A, Horvath E, Bose Dasgupta S, Ashok D, Pieters J, Tacchini-Cottier F, Rolink A, Acha-Orbea H, Finke D (2014) Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc Natl Acad Sci U S A 111:12835–12840
Lehmann FM, von Burg N, Ivanek R, Teufel C, Horvath E, Peter A, Turchinovich G, Staehli D, Eichlisberger T, Gomez de Aguero M, Coto-Llerena M, Prchal-Murphy M, Sexl V, Bentires-Alj M, Mueller C, Finke D (2020) Microbiota-induced tissue signals regulate ILC3-mediated antigen presentation. Nat Commun 11:1794
Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, McNeel DG (2022) Role of B cells as antigen presenting cells. Front Immunol 13:954936
Batista FD, Harwood NE (2009) The who, how and where of antigen presentation to B cells. Nat Rev Immunol 9:15–27
Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA (2006) Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A 103:732–737
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA (2005) Diversity of the human intestinal microbial flora. Science 308:1635–1638
Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D (2011) Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12:1045–1054
Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, Locksley RM (2018) Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 19:1093–1099
Satoh-Takayama N, Kato T, Motomura Y, Kageyama T, Taguchi-Atarashi N, Kinoshita-Daitoku R, Kuroda E, Di Santo JP, Mimuro H, Moro K, Ohno H (2020) Bacteria-induced group 2 innate lymphoid cells in the stomach provide immune protection through induction of IgA. Immunity 52(635–49):e4
Gatto D, Paus D, Basten A, Mackay CR, Brink R (2009) Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity 31:259–269
Chun E, Lavoie S, Fonseca-Pereira D, Bae S, Michaud M, Hoveyda HR, Fraser GL, Gallini Comeau CA, Glickman JN, Fuller MH, Layden BT, Garrett WS (2019) Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 51(871–84):e6
Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T, Setoyama H, Imaoka A, Uematsu S, Akira S, Domino SE, Kulig P, Becher B, Renauld JC, Sasakawa C, Umesaki Y, Benno Y, Kiyono H (2014) Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345:1254009
Chu C, Moriyama S, Li Z, Zhou L, Flamar AL, Klose CSN, Moeller JB, Putzel GG, Withers DR, Sonnenberg GF, Artis D (2018) Anti-microbial functions of group 3 innate lymphoid cells in gut-associated lymphoid tissues are regulated by g-protein-coupled receptor 183. Cell Rep 23:3750–3758
Fachi JL, Sécca C, Rodrigues PB, Mato FCP, Di Luccia B, Felipe JS, Pral LP, Rungue M, Rocha VM, Sato FT, Sampaio U, Clerici MTPS, Rodrigues HG, Câmara NOS, Consonni SR, Vieira AT, Oliveira SC, Mackay CR, Layden BT, Bortoluci KR, Colonna M, Vinolo MAR (2020) Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J Exp Med 217(3):jem.20190489. https://doi.org/10.1084/jem.20190489
Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, Baumler AJ (2008) T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun 76:2008–2017
Kim MH, Taparowsky EJ, Kim CH (2015) Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity 43:107–119
van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labao-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O’Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H (2014) Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508:123–127
Lin YD, Arora J, Diehl K, Bora SA, Cantorna MT (2019) Vitamin D is required for ILC3 derived IL-22 and protection from Citrobacter rodentium infection. Front Immunol 10:1
Chen J, Waddell A, Lin YD, Cantorna MT (2015) Dysbiosis caused by vitamin D receptor deficiency confers colonization resistance to Citrobacter rodentium through modulation of innate lymphoid cells. Mucosal Immunol 8:618–626
He L, Zhou M, Li YC (2019) Vitamin D/vitamin D receptor signaling is required for normal development and function of group 3 innate lymphoid cells in the gut. iScience 17:119–31
Sun J (2010) Vitamin D and mucosal immune function. Curr Opin Gastroenterol 26:591–595
Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X (2012) Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol 12:57
Jadhav P, Jiang Y, Jarr K, Layton C, Ashouri JF, Sinha SR (2020) Efficacy of dietary supplements in inflammatory bowel disease and related autoimmune diseases. Nutrients 12(7):2156. https://doi.org/10.3390/nu12072156
Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG, Howard JK, Parkhill J, MacDonald TT, Lord GM (2012) The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 37:674–684
Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F (2011) IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 208:1127–1133
Song C, Lee JS, Gilfillan S, Robinette ML, Newberry RD, Stappenbeck TS, Mack M, Cella M, Colonna M (2015) Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med 212:1869–1882
Li J, Shi W, Sun H, Ji Y, Chen Y, Guo X, Sheng H, Shu J, Zhou L, Cai T, Qiu J (2019) Activation of DR3 signaling causes loss of ILC3s and exacerbates intestinal inflammation. Nat Commun 10:3371
Zhou L, Zhou W, Joseph AM, Chu C, Putzel GG, Fang B, Teng F, Lyu M, Yano H, Andreasson KI, Mekada E, Eberl G, Sonnenberg GF (2022) Group 3 innate lymphoid cells produce the growth factor HB-EGF to protect the intestine from TNF-mediated inflammation. Nat Immunol 23:251–261
Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, Zhou L, Zhou J, Zhang W, Bank JRILC, Shen Z, Fox JG, Sockolow RE, Laufer TM, Fan Y, Eberl G, Withers DR, Sonnenberg GF (2022) ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 610:744–751
Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, Wherry EJ, Koni PA, Bushman FD, Elson CO, Eberl G, Artis D, Sonnenberg GF (2013) Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498:113–117
Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, Bemelman WA, Diefenbach A, Blom B, Spits H (2015) Interleukin-12 and -23 control plasticity of cd127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43:146–160
Bauche D, Joyce-Shaikh B, Jain R, Grein J, Ku KS, Blumenschein WM, Ganal-Vonarburg SC, Wilson DC, McClanahan TK, Malefyt RW, Macpherson AJ, Annamalai L, Yearley JH, Cua DJ (2018) LAG3(+) Regulatory T cells restrain interleukin-23-producing CX3CR1(+) gut-resident macrophages during group 3 innate lymphoid cell-driven colitis. Immunity 49(342–52):e5
Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M (2014) Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343:1249288
Zhou L, Chu C, Teng F, Bessman NJ, Goc J, Santosa EK, Putzel GG, Kabata H, Kelsen JR, Baldassano RN, Shah MA, Sockolow RE, Vivier E, Eberl G, Smith KA, Sonnenberg GF (2019) Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 568:405–409
Bergmann H, Roth S, Pechloff K, Kiss EA, Kuhn S, Heikenwalder M, Diefenbach A, Greten FR, Ruland J (2017) Card9-dependent IL-1beta regulates IL-22 production from group 3 innate lymphoid cells and promotes colitis-associated cancer. Eur J Immunol 47:1342–1353
Chan IH, Jain R, Tessmer MS, Gorman D, Mangadu R, Sathe M, Vives F, Moon C, Penaflor E, Turner S, Ayanoglu G, Chang C, Basham B, Mumm JB, Pierce RH, Yearley JH, McClanahan TK, Phillips JH, Cua DJ, Bowman EP, Kastelein RA, LaFace D (2014) Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol 7:842–856
Wang S, Qu Y, Xia P, Chen Y, Zhu X, Zhang J, Wang G, Tian Y, Ying J, Fan Z (2020) Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res 30:610–622
Huang J, Lee HY, Zhao X, Han J, Su Y, Sun Q, Shao J, Ge J, Zhao Y, Bai X, He Y, Wang X, Wang X, Dong C (2021) Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity 54(673–86):e4
Gronke K, Hernandez PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C, Amann L, Schumacher F, Glatt H, Triantafyllopoulou A, Diefenbach A (2019) Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566:249–253
Goc J, Lv M, Bessman NJ, Flamar AL, Sahota S, Suzuki H, Teng F, Putzel GG, Bank JRILC, Eberl G, Withers DR, Arthur JC, Shah MA, Sonnenberg GF (2021) Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 184(5015–30):e16
Fu W, Wang W, Zhang J, Zhao Y, Chen K, Wang Y, Zhang J, Xiong Y, Guo X, Ding S (2022) Dynamic change of circulating innate and adaptive lymphocytes subtypes during a cascade of gastric lesions. J Leukoc Biol 112:931–938
Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjosberg JM, Spits H (2013) Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 14:221–229
Li J, Doty AL, Iqbal A, Glover SC (2016) The differential frequency of Lineage(-)CRTH2(-)CD45(+)NKp44(-)CD117(-)CD127(+)ILC subset in the inflamed terminal ileum of patients with Crohn’s disease. Cell Immunol 304–305:63–68
Gwela A, Siddhanathi P, Chapman RW, Travis S, Powrie F, Arancibia-Carcamo CV, Geremia A (2017) Th1 and innate lymphoid cells accumulate in primary sclerosing cholangitis-associated inflammatory bowel disease. J Crohns Colitis 11:1124–1134
Camelo A, Barlow JL, Drynan LF, Neill DR, Ballantyne SJ, Wong SH, Pannell R, Gao W, Wrigley K, Sprenkle J, McKenzie AN (2012) Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol 47:1198–1211
Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D (2015) IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A 112:10762–10767
Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F (2013) Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 210:917–931
Bando JK, Gilfillan S, Di Luccia B, Fachi JL, Sécca C, Cella M, Colonna M (2020) ILC2s are the predominant source of intestinal ILC-derived IL-10. J Exp Med 217(2):e20191520. https://doi.org/10.1084/jem.20191520
Seehus CR, Kadavallore A, Torre B, Yeckes AR, Wang Y, Tang J, Kaye J (2017) Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun 8:1900
Xuan X, Zhou J, Tian Z, Lin Y, Song J, Ruan Z, Ni B, Zhao H, Yang W (2020) ILC3 cells promote the proliferation and invasion of pancreatic cancer cells through IL-22/AKT signaling. Clin Transl Oncol 22:563–575
Liu Y, Song Y, Lin D, Lei L, Mei Y, Jin Z, Gong H, Zhu Y, Hu B, Zhang Y, Zhao L, Teo HY, Qiu J, Jiang W, Dong C, Wu D, Huang Y, Liu H (2019) NCR(-) group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine 41:333–344
Salimi M, Wang R, Yao X, Li X, Wang X, Hu Y, Chang X, Fan P, Dong T, Ogg G (2018) Activated innate lymphoid cell populations accumulate in human tumour tissues. BMC Cancer 18:341
Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C (2008) NKG2D ligands: key targets of the immune response. Trends Immunol 29:397–403
Kramer B, Goeser F, Lutz P, Glassner A, Boesecke C, Schwarze-Zander C, Kaczmarek D, Nischalke HD, Branchi V, Manekeller S, Huneburg R, van Bremen T, Weismuller T, Strassburg CP, Rockstroh JK, Spengler U, Nattermann J (2017) Compartment-specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLoS Pathog 13:e1006373
Serafini N, Klein Wolterink RG, Satoh-Takayama N, Xu W, Vosshenrich CA, Hendriks RW, Di Santo JP (2014) Gata3 drives development of RORgammat+ group 3 innate lymphoid cells. J Exp Med 211:199–208
Ward RL (1996) Mechanisms of protection against rotavirus in humans and mice. J Infect Dis 174(Suppl 1):S51–S58
Karst SM, Tibbetts SA (2016) Recent advances in understanding norovirus pathogenesis. J Med Virol 88:1837–1843
Hernandez PP, Mahlakoiv T, Yang I, Schwierzeck V, Nguyen N, Guendel F, Gronke K, Ryffel B, Hoelscher C, Dumoutier L, Renauld JC, Suerbaum S, Staeheli P, Diefenbach A (2015) Interferon-lambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat Immunol 16:698–707
Neil JA, Matsuzawa-Ishimoto Y, Kernbauer-Holzl E, Schuster SL, Sota S, Venzon M, Dallari S, Galvao Neto A, Hine A, Hudesman D, Loke P, Nice TJ, Cadwell K (2019) IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat Microbiol 4:1737–1749
Crakes KR, Jiang G (2019) Gut microbiome alterations during HIV/SIV infection: Implications for HIV Cure. Front Microbiol 10:1104
Li H, Richert-Spuhler LE, Evans TI, Gillis J, Connole M, Estes JD, Keele BF, Klatt NR, Reeves RK (2014) Hypercytotoxicity and rapid loss of NKp44+ innate lymphoid cells during acute SIV infection. PLoS Pathog 10:e1004551
Muhl H, Bachmann M (2019) IL-18/IL-18BP and IL-22/IL-22BP: two interrelated couples with therapeutic potential. Cell Signal 63:109388
Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF (2013) IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 182:1286–1296
Withers DR, Hepworth MR (2017) Group 3 innate lymphoid cells: communications hubs of the intestinal immune system. Front Immunol 8:1298
Acknowledgements
We thank Dr. Peter Burrows for English proofreading and suggestion on the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI (20KK0360 for N. S.-T.) and Japan Agency for Medical Research and Development Care Research for Evolutional Science Technology (21gm6310027h for N. S.-T.).
Author information
Authors and Affiliations
Contributions
Ayana Mori and Naoko Satoh-Takayama wrote the manuscript. Hiroshi Ohno provided his support and critical advice. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Article Collection on Immunopathology of Barrier Function – Guest Editor: Koji Hase & Hiroshi Ohno
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mori, A., Ohno, H. & Satoh-Takayama, N. Disease pathogenesis and barrier functions regulated by group 3 innate lymphoid cells. Semin Immunopathol (2024). https://doi.org/10.1007/s00281-024-01000-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00281-024-01000-1