Abstract
Purpose
Apalutamide plus androgen-deprivation therapy (ADT) has been approved for treatment of patients with metastatic castration-sensitive prostate cancer (mCSPC) based on data from phase 3 TITAN study. This analysis was conducted to describe pharmacokinetics of apalutamide and N-desmethyl-apalutamide and explore relationships between apalutamide exposure and selected clinical efficacy and safety observations.
Methods
1052 patients were randomized to apalutamide + ADT (n = 525) or placebo + ADT (n = 527). A previously developed population pharmacokinetic model was applied. Cox regression analysis investigated the relationships between apalutamide exposure and overall survival (OS; n = 1004) and radiographic progression-free survival (rPFS; n = 1003). Logistic regression analysis assessed the relationships between apalutamide exposure and selected clinically relevant adverse events (n = 1051).
Results
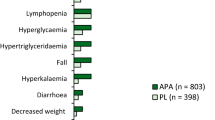
Apalutamide + ADT treatment was efficacious in extending rPFS and OS versus placebo + ADT. Within a relatively narrow apalutamide exposure range (coefficient of variation: 22%), no statistical association was detected between rPFS, OS and apalutamide exposure quartiles. Incidence of skin rash and pruritus increased significantly with increasing apalutamide exposure.
Conclusions
Differences in apalutamide exposure were not associated with clinically relevant differences in rPFS or OS in patients with mCSPC. Patients with increased apalutamide exposure are more likely to develop skin rash and pruritus. Dose reductions may improve these adverse events, based on an individual risk–benefit approach.



Similar content being viewed by others
Availability of data and material
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access [YODA] Project site at http://yoda.yale.edu.
References
Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10(2):63
Damodaran S, Lang JM, Jarrard DF (2019) Targeting metastatic hormone sensitive prostate cancer: chemohormonal therapy and new combinatorial approaches. J Urol 201(5):876–885. https://doi.org/10.1097/ju.0000000000000117
Hahn AW, Higano CS, Taplin ME, Ryan CJ, Agarwal N (2018) Metastatic castration-sensitive prostate cancer: optimizing patient selection and treatment. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Ann Meet 38:363–371. https://doi.org/10.1200/edbk_200967
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O’Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet (London, England) 387(10024):1163–1177. https://doi.org/10.1016/s0140-6736(15)01037-5
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377(4):352–360. https://doi.org/10.1056/NEJMoa1704174
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, Matheson D, Millman R, Attard G, Chowdhury S, Cross WR, Gillessen S, Parker CC, Russell JM, Berthold DR, Brawley C, Adab F, Aung S, Birtle AJ, Bowen J, Brock S, Chakraborti P, Ferguson C, Gale J, Gray E, Hingorani M, Hoskin PJ, Lester JF, Malik ZI, McKinna F, McPhail N, Money-Kyrle J, O’Sullivan J, Parikh O, Protheroe A, Robinson A, Srihari NN, Thomas C, Wagstaff J, Wylie J, Zarkar A, Parmar MKB, Sydes MR (2017) Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377(4):338–351. https://doi.org/10.1056/NEJMoa1702900
Smith MR, Antonarakis ES, Ryan CJ, Berry WR, Shore ND, Liu G, Alumkal JJ, Higano CS, Chow Maneval E, Bandekar R, de Boer CJ, Yu MK, Rathkopf DE (2016) Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol 70(6):963–970. https://doi.org/10.1016/j.eururo.2016.04.023
Borno HT, Small EJ (2019) Apalutamide and its use in the treatment of prostate cancer. Fut Oncol (Lond, Engl) 15(6):591–599. https://doi.org/10.2217/fon-2018-0546
Erleada [Internet]. Eur. Med. Agency. 2018. . Available from: https://www.emaeuropaeu/en/medicines/human/EPAR/erleada. Accessed November 15, 2019
ERLEADATM (apalutamide) prescribing information. [Internet]. 2019. . Available from: https https://www.accessdatafdagov/drugsatfda_docs/label/2019/210951s001lblpdf. Accessed December 14, 2019
US Food and Drug administration. FDA approves apalutamide for metastatic castration-sensitive prostate cancer. FDA [Internet]. 2019. Available from: http: // wwwfdagov/drugs/resources-information-approved-drugs/fda-approves-apalutamide-metastatic-castration-sensitive-prostate-cancer Accessed November 15,2019
Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, Jarrard DF, Kibel AS, Morgan TM, Morgans AK, Oh WK, Resnick MJ, Zietman AL, Cookson MS (2021) Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J Urol 205(1):14–21. https://doi.org/10.1097/JU.0000000000001375
de Vries R, Jacobs F, Mannens G, Snoeys J, Cuyckens F, Chien C, Ward P (2019) Apalutamide absorption, metabolism, and excretion in healthy men, and enzyme reaction in human hepatocytes. Drug Metab Dispos 47(5):453–464. https://doi.org/10.1124/dmd.118.084517
Pérez-Ruixo C, Pérez-Blanco JS, Chien C, Yu M, Ouellet D, Pérez-Ruixo JJ, Ackaert O (2020) Population pharmacokinetics of apalutamide and its active metabolite N-desmethyl-apalutamide in healthy and castration-resistant prostate cancer subjects. Clin Pharmacokinet 59(2):229–244. https://doi.org/10.1007/s40262-019-00808-7
Chi KN, Agarwal N, Bjartell A, Chung BH, de Santana P, Gomes AJ, Given R, Juárez Soto Á, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Deprince K, Naini V, Li J, Cheng S, Yu MK, Zhang K, Larsen JS, McCarthy S, Chowdhury S (2019) Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 381(1):13–24. https://doi.org/10.1056/NEJMoa1903307
Chi KN, Chowdhury S, Bjartell A, Chung BH, de Santana P, Gomes AJ, Given R, Juárez A, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Brookman-May S, Mundle SD, McCarthy SA, Larsen JS, Sun W, Bevans KB, Zhang K, Bandyopadhyay N, Agarwal N (2021) Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind Phase III TITAN Study. J Clin Oncol. https://doi.org/10.1200/jco.20.03488
Agarwal N, McQuarrie K, Bjartell A, Chowdhury S, de Santana P, Gomes AJ, Chung BH, Özgüroğlu M, Juárez Soto Á, Merseburger AS, Uemura H, Ye D, Given R, Cella D, Basch E, Miladinovic B, Dearden L, Deprince K, Naini V, Lopez-Gitlitz A, Chi KN (2019) Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol 20(11):1518–1530. https://doi.org/10.1016/s1470-2045(19)30620-5
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Tu E, Vera-Badillo F, Williams SG, Yip S, Zhang AY, Zielinski RR, Sweeney CJ (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381(2):121–131. https://doi.org/10.1056/NEJMoa1903835
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, Lopez-Gitlitz A, Trudel GC, Espina BM, Shu Y, Park YC, Rackoff WR, Yu MK, Small EJ (2018) Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 378(15):1408–1418. https://doi.org/10.1056/NEJMoa1715546
Perez-Ruixo C, Ackaert O, Ouellet D, Chien C, Uemura H, Olmos D, Mainwaring P, Lee JY, Yu MK, Perez-Ruixo JJ, Smith MR, Small EJ (2020) Efficacy and safety exposure-response relationships of apalutamide in patients with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res 26(17):4460–4467. https://doi.org/10.1158/1078-0432.Ccr-20-1041
Saad F, Cella D, Basch E, Hadaschik BA, Mainwaring PN, Oudard S, Graff JN, McQuarrie K, Li S, Hudgens S, Lawson J, Lopez-Gitlitz A, Yu MK, Smith MR, Small EJ (2018) Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol 19(10):1404–1416. https://doi.org/10.1016/s1470-2045(18)30456-x
Beal S SL, Boeckmann A, Bauer R, editors. NONMEM 7.2.0 Users Guides. Ellicott City, MD: Icon Development Solutions; 1989–2011.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. . Available from: https: // wwwr-projectorg/ Accessed November 15, 2019
Li Z, Meredith MP, Hoseyni MS (2001) A method to assess the proportion of treatment effect explained by a surrogate endpoint. Stat Med 20(21):3175–3188. https://doi.org/10.1002/sim.984
US Food and Drug administration. Guidance for industry: Exposure-Response Relationships — Study Design, Data Analysis, and Regulatory Applications [Internet]. 2003. Available from: https :// wwwfdagov/media/71277/download Accessed November 15,2019
van Nuland M, Bergman AM, Rosing H, de Vries N, Huitema ADR, Beijnen JH (2019) Exposure-response assessment of enzalutamide and its major metabolites in a real-world cohort of patients with metastatic castration-resistant prostate cancer. Pharmacotherapy 39(12):1137–1145. https://doi.org/10.1002/phar.2339
Joulia ML, Carton E, Jouinot A, Allard M, Huillard O, Khoudour N, Peyromaure M, Zerbib M, Schoemann AT, Vidal M, Goldwasser F, Alexandre J, Blanchet B (2020) Pharmacokinetic/pharmacodynamic relationship of enzalutamide and its active metabolite N-desmethyl enzalutamide in metastatic castration-resistant prostate cancer patients. Clin Genitourin Cancer 18(2):155–160. https://doi.org/10.1016/j.clgc.2019.05.020
Benoist GE, van Oort IM, Burger DM, Mehra N, van Erp NP (2020) The impact of patient characteristics on enzalutamide pharmacokinetics and how this relates to treatment toxicity and efficacy in metastatic prostate cancer patients. Cancer Chemother Pharmacol 85(4):753–760. https://doi.org/10.1007/s00280-020-04039-7
Rathkopf DE, Morris MJ, Fox JJ, Danila DC, Slovin SF, Hager JH, Rix PJ, Maneval EC, Chen I, Gönen M, Fleisher M, Larson SM, Sawyers CL, Scher HI (2013) Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol 31(28):3525–3530. https://doi.org/10.1200/jco.2013.50.1684
Acknowledgements
The authors would like to thank the patients, investigators, and their medical, nursing and laboratory staff who participated in the clinical studies included in the present work. Editorial assistance was provided by Akshada Deshpande, PhD (SIRO Clinpharm Pvt Ltd) and Namit Ghildyal, PhD (Janssen Global Services, LLC), funded by Janssen Global Services, LLC.
Funding
The clinical studies were supported by research funding from Janssen Research & Development, and the analyses presented here were supported by Janssen Research & Development.
Author information
Authors and Affiliations
Contributions
HT and OA were involved in development of methodology, analysis and interpretation of data and provided administrative, technical, or material support. CC contributed to the conception of the study and interpretation of data. ALG contributed to the conception of the study and supervised the study. SM contributed to the conception of the study. CPR contributed to the analysis and interpretation of data. LK, KC, SC, and NA were involved in acquisition of data. JJPR was involved in development of methodology and analysis and interpretation of data. All authors contributed to drafting, review and/or revisions of the manuscript, and all authors approved the final version of the manuscript for submission and publication.
Corresponding author
Ethics declarations
Conflict of interest
HT, OA, CC, ALG, SM, JJPR, and CPR: Employees of Janssen Research & Development at time of conduct of the study; CC, ALG, SM, and JJPR: Stock owners of Johnson & Johnson at time of conduct of the study. NA: Consultancy: Astellas, Astra Zeneca, Bayer, Bristol Myers Squibb, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Janssen, Merck, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics; Research funding: Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. KC: Honoraria: Novartis, Janssen, Astellas, Sanofi, Astra Zeneca, Roche, Daiichi Sankyo, Pfizer, and Point Biopharma; and Research funding: Novartis, Janssen, Astellas, Sanofi, Astra Zeneca, and Roche. SC: Consultancy: Clovis Oncology, Astellas Pharma, Bayer, Pfizer, and Janssen-Cilag; Honoraria: Clovis Oncology, and Novartis; Speakers’ Bureau: Pfizer; Research funding: Sanofi/Aventis. LK: Stock Owner: Swan Valley Medical; Honoraria: Astellas, Bayer, Janssen, Pfizer and Dendreon; Consultancy: 3D Biopsy, Astellas, Astra-Zeneca, Bayer, Dendreon, Ferring, Janssen, Pfizer, and Vaxiion; Speakers’ Bureau: Astellas, Bayer, Janssen, Pfizer, and Clovis; Travel, Accommodations, Expenses: Astellas, Bayer, Janssen, Pfizer, and Dendreon; Research funding: Astellas, Astra Zeneca, Bayer, BioXcel Therapeutics, Bristol Meyers Squibb, CU Optics, CUSP, Dendreon, Epizyme, Exact Sciences, Ferring, FKD, Genentech/Roche, GenomeDx, Genomic Health, Janssen, Merck, Myovant, Nucleix, OncoCell MDx, Pfizer, Pharmtech/Veru, Precision Med, QED Therapeutics, Siemens, Urogen, and Vaxiion.
Ethical approval
The study was conducted in accordance with principles for human experimentation as defined in the Declaration of Helsinki and was approved by the Human Investigational Review Board of each study center and by the Competent Authority of each country.
Consent to participate
Informed consent was obtained from each subject before enrollment in the study, after being advised of the potential risks and benefits, as well as the investigational nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
T’jollyn, H., Ackaert, O., Chien, C. et al. Efficacy and safety exposure–response relationships of apalutamide in patients with metastatic castration-sensitive prostate cancer: results from the phase 3 TITAN study. Cancer Chemother Pharmacol 89, 629–641 (2022). https://doi.org/10.1007/s00280-022-04427-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04427-1