Abstract
Purpose
S-1 plus cisplatin therapy is the recommended standard first-line regimen for human epidermal growth factor receptor 2 (HER-2)-negative advanced unresectable or recurrent gastric cancer (AGC) in the Japanese Gastric Cancer Treatment Guidelines. By contrast, capecitabine plus cisplatin (XP) therapy has been second-line therapy for these patients. This prospective study aimed to evaluate the efficacy and safety of XP as a first-line regimen for HER2-negative patients with AGC.
Methods
In this multicenter, open-label, phase II study, patients received cisplatin (80 mg/m2 i.v. day 1) plus capecitabine (1000 mg/m2 orally, twice daily, days 1–14) at 3 week intervals until disease progression or non-continuation for various reasons. The primary endpoint was overall response rate; secondary endpoints included progression-free survival (PFS), overall survival (OS), and toxicity profiles.
Results
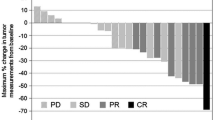
Thirty-six patients with HER2-negative AGC were enrolled in this study. Of these, 16 patients with evaluable lesions were assessable for efficacy and 36 were assessable for toxicity. One patient achieved a complete response and five partial responses. The overall response rate was 37.5% [95% confidence interval (CI) 13.7–61.2%] calculated on an intention-to-treat basis. The median PFS and median OS were 5.2 months (95% CI 4.2–6.2 months) and 16.9 months (95% CI 5.8–27.9 months), respectively. Treatment-related adverse events were generally mild; the most common grade 3/4 adverse event was neutropenia (27.8%), followed by anorexia (19.4%), leucopenia (16.7%), anemia (16.7%), and nausea (13.9%).
Conclusion
XP as first-line therapy is effective and well tolerated by patients with HER2-negative AGC.


Similar content being viewed by others
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403. doi:10.1016/j.ejca.2012.12.027
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. doi:10.1016/S1470-2045(08)70035-4
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20(4):666–673. doi:10.1093/annonc/mdn717
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK, Investigators TT (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28(9):1547–1553. doi:10.1200/JCO.2009.25.4706
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29(30):3968–3976. doi:10.1200/JCO.2011.36.2236
Narahara H, Iishi H, Imamura H, Tsuburaya A, Chin K, Imamoto H, Esaki T, Furukawa H, Hamada C, Sakata Y (2011) Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer 14(1):72–80. doi:10.1007/s10120-011-0009-5
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1):1–19. doi:10.1007/s10120-016-0622-4
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu J, Ohtsu A, Group GOSGotJCO (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10 (11):1063–1069. doi:10.1016/S1470-2045(09)70259-1
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 26(1):141–148. doi:10.1093/annonc/mdu472
Kim GM, Jeung HC, Rha SY, Kim HS, Jung I, Nam BH, Lee KH, Chung HC (2012) A randomized phase II trial of S-1-oxaliplatin versus capecitabine–oxaliplatin in advanced gastric cancer. Eur J Cancer 48(4):518–526. doi:10.1016/j.ejca.2011.12.017
Li Q, Wen F, Zhou C, Qiu M, Liu J, Chen J, Yi C, Li Z, Luo D, Xu F, Cai X, Bi F, China WCGOGo (2017) Prospective randomized phase II study of FOLFIRI versus FOLFOX7 in advanced gastric adenocarcinoma: a Chinese Western Cooperative Gastrointestinal Oncology Group study. Oncotarget. doi:10.18632/oncotarget.18426
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 1 June 2016
Yamaguchi K, Sawaki A, Doi T, Satoh T, Yamada Y, Omuro Y, Nishina T, Boku N, Chin K, Hamamoto Y, Takiuchi H, Komatsu Y, Saji S, Koizumi W, Miyata Y, Sato A, Baba E, Tamura T, Abe T, Ohtsu A (2013) Efficacy and safety of capecitabine plus cisplatin in Japanese patients with advanced or metastatic gastric cancer: subset analyses of the AVAGAST study and the ToGA study. Gastric Cancer 16(2):175–182. doi:10.1007/s10120-012-0167-0
Seol YM, Song MK, Choi YJ, Kim GH, Shin HJ, Song GA, Chung JS, Cho GJ (2009) Oral fluoropyrimidines (capecitabine or S-1) and cisplatin as first line treatment in elderly patients with advanced gastric cancer: a retrospective study. Jpn J Clin Oncol 39(1):43–48. doi:10.1093/jjco/hyn119
Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, Kim BS, Lee JS (2002) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 13(12):1893–1898
Cunningham D, Okines AF, Ashley S (2010) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 362(9):858–859. doi:10.1056/NEJMc0911925
Acknowledgements
We gratefully thank the staff members in the YCU Center for Novel and Exploratory Clinical Trials (Y-NEXT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sato, K., Kunisaki, C., Kosaka, T. et al. Multicenter phase II study of capecitabine plus cisplatin as first-line therapy for human epidermal growth factor receptor 2-negative advanced gastric cancer: Yokohama Clinical Oncology Group Study YCOG1107. Cancer Chemother Pharmacol 80, 939–943 (2017). https://doi.org/10.1007/s00280-017-3430-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3430-6