Abstract
Purpose
Axitinib is a potent and selective inhibitor of vascular endothelial growth factor receptors 1–3, approved for second-line treatment of advanced renal cell carcinoma (RCC). Preclinical studies did not indicate potential for axitinib-induced delayed cardiac repolarization.
Methods
The effect of axitinib on corrected QT (QTc) prolongation was evaluated with one-stage concentration–QTc response modeling using data from a definitive randomized crossover QT phase I study in healthy volunteers administered one single 5-mg axitinib dose alone or in the presence of steady-state ketoconazole (400 mg once daily).
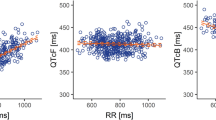
Results
Axitinib and ketoconazole had opposite effects on heart rate: Axitinib lowered it, ketoconazole raised it. The final analysis showed a flat relationship between QTc and axitinib concentration (slope −0.0314 ms·mL/ng) for axitinib alone. Mean highest placebo-matched change from baseline in QTc was −3.0 [90 % confidence interval (CI) −5.4, −0.6] ms. At supratherapeutic axitinib exposures achieved with potent cytochrome P450 3A4/5 inhibition by ketoconazole, the model predicted mean QTc change of 6.5 (90 % CI 4.4–8.5) ms. The slope population mean estimate was −0.331 (95 % CI −0.860, 0.198) ms·mL/µg for ketoconazole alone and 0.0725 (0.0445–0.1005) ms·mL/ng for axitinib in the presence of ketoconazole. The results were then compared with those obtained based on more widely used Fridericia’s, Bazett’s, and study-specific correction methods.
Conclusions
Since axitinib plasma concentrations observed in this study exceeded the range of concentrations observed in patients with RCC at the highest approved clinical dose (10 mg twice daily), axitinib was not associated with clinically significant QTc prolongation in target populations.



Similar content being viewed by others
References
Roden DM (2008) Cellular basis of drug-induced torsades de pointes. Br J Pharmacol 154:1502–1507. doi:10.1038/bjp.2008.238
U.S. Department of Health and Human Services Food and Drug Administration (2005) Guidance for industry, E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129357.pdf. Accessed 4 Feb 2013
Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O’Connor P, Shalinsky DR, Bender SL (2008) Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 14:7272–7283. doi:10.1158/1078-0432.CCR-08-0652
Inlyta® (axitinib) prescribing information (2012). http://labeling.pfizer.com/ShowLabeling.aspx?id=759. Accessed 22 Jan 2013
Rugo HS, Herbst RS, Liu G, Park JW, Kies MS, Steinfeldt HM, Pithavala YK, Reich SD, Freddo JL, Wilding G (2005) Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 23:5474–5483. doi:10.1200/JCO.2005.04.192
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou Y-C, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378:1931–1939. doi:10.1016/S0140-6736(11)61613-9
Di Lorenzo G, Porta C, Bellmunt J, Sternberg C, Kirkali Z, Staehler M, Joniau S, Montorsi F, Buonerba C (2011) Toxicities of targeted therapy and their management in kidney cancer. Eur Urol 59:526–540. doi:10.1016/j.eururo.2011.01.002
Chen Y, Tortorici MA, Garrett M, Hee B, Klamerus KJ, Pithavala YK (2013) Clinical pharmacology of axitinib. Clin Pharmacokinet 52:713–725. doi:10.1007/s40262-013-0068-3
Pithavala YK, Tong W, Mount J, Rahavendran SV, Garrett M, Hee B, Selaru P, Sarapa N, Klamerus KJ (2012) Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Invest New Drugs 30:273–281. doi:10.1007/s10637-010-9511-6
Tornoe CW, Garnett CE, Wang Y, Florian J, Li M, Gobburu JV (2011) Creation of a knowledge management system for QT analyses. J Clin Pharmacol 51:1035–1042. doi:10.1177/0091270010378408
Garnett CE, Zhu H, Malik M, Fossa AA, Zhang J, Badilini F, Li J, Darpo B, Sager P, Rodriguez I (2012) Methodologies to characterize the QT/corrected QT interval in the presence of drug-induced heart rate changes or other autonomic effects. Am Heart J 163:912–930. doi:10.1016/j.ahj.2012.02.023
Sweeney KR, Gastonguay MR, Benincosa L, Cronenberger CL, Glue P, Malhotra BK (2010) Exposure–response modeling and clinical trial simulation of the effect of tolterodine on QT intervals in healthy volunteers. Drug Discov Ther 4:44–53
Desai M, Li L, Desta Z, Malik M, Flockhart D (2003) Variability of heart rate correction methods for the QT interval. Br J Clin Pharmacol 55:511–517. doi:10.1046/j.1365-2125.2003.01791.x
Bello CL, Mulay M, Huang X, Patyna S, Dinolfo M, Levine S, Van Vugt A, Toh M, Baum C, Rosen L (2009) Electrocardiographic characterization of the QTc interval in patients with advanced solid tumors: pharmacokinetic–pharmacodynamic evaluation of sunitinib. Clin Cancer Res 15:7045–7052. doi:10.1158/1078-0432.CCR-09-1521
Chien JY, Lucksiri A, Ernest CS 2nd, Gorski JC, Wrighton SA, Hall SD (2006) Stochastic prediction of CYP3A-mediated inhibition of midazolam clearance by ketoconazole. Drug Metab Dispos 34:1208–1219. doi:10.1124/dmd.105.008730
Tortorici MA, Toh M, Rahavendran SV, Labadie RR, Alvey CW, Marbury T, Fuentes E, Green M, Ni G, Hee B, Pithavala YK (2011) Influence of mild and moderate hepatic impairment on axitinib pharmacokinetics. Invest New Drugs 29:1370–1380. doi:10.1007/s10637-010-9477-4
Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornoe CW, Wang Y, Zhu H, Gobburu JV (2008) Concentration–QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18. doi:10.1177/0091270007307881
Russell T, Riley SP, Cook JA, Lalonde RL (2008) A perspective on the use of concentration–QT modeling in drug development. J Clin Pharmacol 48:9–12. doi:10.1177/0091270007311115
Chapel S, Hutmacher MM, Bockbrader H, de Greef R, Lalonde RL (2011) Comparison of QTc data analysis methods recommended by the ICH E14 guidance and exposure–response analysis: case study of a thorough QT study of asenapine. Clin Pharmacol Ther 89:75–80. doi:10.1038/clpt.2010.220
Darpo B, Garnett C, Benson CT, Keirns J, Leishman D, Malik M, Mehrotra N, Prasad K, Riley S, Rodriguez I, Sager P, Sarapa N, Wallis R (2014) Cardiac Safety Research Consortium: Can the thorough QT/QTc study be replaced by early QT assessment in routine clinical pharmacology studies? Scientific update and a research proposal for a path forward. Am Heart J 168:262–272. doi:10.1016/j.ahj.2014.06.003
Darpo B, Sarapa N, Garnett C, Benson C, Dota C, Ferber G, Jarugula V, Johannesen L, Keirns J, Krudys K, Ortemann-Renon C, Riley S, Rogers-Subramaniam D, Stockbridge N (2014) The IQ-CSRC prospective clinical phase 1 study: “Can early QT assessment using exposure response analysis replace the thorough QT study?”. Ann Noninvasive Electrocardiol 19:70–81. doi:10.1111/anec.12128
Darpo B, Nebout T, Sager PT (2006) Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use E14 guideline. J Clin Pharmacol 46:498–507. doi:10.1177/0091270006286436
Zhu H, Wang Y, Gobburu JV, Garnett CE (2010) Considerations for clinical trial design and data analyses of thorough QT studies using drug–drug interaction. J Clin Pharmacol 50:1106–1111. doi:10.1177/0091270009358710
Rock EP, Finkle J, Fingert HJ, Booth BP, Garnett CE, Grant S, Justice RL, Kovacs RJ, Kowey PR, Rodriguez I, Sanhai WR, Strnadova C, Targum SL, Tsong Y, Uhl K, Stockbridge N (2009) Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J 157:827–836. doi:10.1016/j.ahj.2009.02.020
Curigliano G, Spitaleri G, Fingert HJ, de Braud F, Sessa C, Loh E, Cipolla C, De Pas T, Goldhirsch A, Shah R (2008) Drug-induced QTc interval prolongation: a proposal towards an efficient and safe anticancer drug development. Eur J Cancer 44:494–500. doi:10.1016/j.ejca.2007.10.001
Malhotra BK, Glue P, Sweeney K, Anziano R, Mancuso J, Wicker P (2007) Thorough QT study with recommended and supratherapeutic doses of tolterodine. Clin Pharmacol Ther 81:377–385. doi:10.1038/sj.clpt.6100089
Li J, Birmingham B, Mosqueda-Garcia R, Sander N, Newbold P, Sager P (2008) Evaluation of drug-induced QT/QTc prolongation in the presence of drug inducted changes in heart rate using population PK/PD modeling approach: sibenadet experience. In: American conference on pharmacometrics (ACoP) annual meeting, Tucson, AZ., Mar 9–12, 2008
Kosoglou T, Salfi M, Lim JM, Batra VK, Cayen MN, Affrime MB (2000) Evaluation of the pharmacokinetics and electrocardiographic pharmacodynamics of loratadine with concomitant administration of ketoconazole or cimetidine. Br J Clin Pharmacol 50:581–589. doi:10.1046/j.1365-2125.2000.00290.x
Chaikin P, Gillen MS, Malik M, Pentikis H, Rhodes GR, Roberts DJ (2005) Co-administration of ketoconazole with H1-antagonists ebastine and loratadine in healthy subjects: pharmacokinetic and pharmacodynamic effects. Br J Clin Pharmacol 59:346–354. doi:10.1111/j.1365-2125.2005.02348.x
Robert M, Salva M, Segarra R, Pavesi M, Esbri R, Roberts D, Golor G (2007) The prokinetic cinitapride has no clinically relevant pharmacokinetic interaction and effect on QT during coadministration with ketoconazole. Drug Metab Dispos 35:1149–1156. doi:10.1124/dmd.106.010835
Sarapa N, Britto MR (2008) Challenges of characterizing proarrhythmic risk due to QTc prolongation induced by nonadjuvant anticancer agents. Expert Opin Drug Saf 7:305–318. doi:10.1517/14740338.7.3.305
Acknowledgments
This study was sponsored by Pfizer Inc. Medical writing support was funded by Pfizer Inc and provided by Mariko Nagashima, PhD, of Engage Scientific Solutions (Southport, CT, USA).
Conflict of interest
Ana Ruiz-Garcia, Brett E. Houk, and Yazdi K. Pithavala are employees of and own stock in Pfizer Inc. Michael A. Tortorici, who was employed by and owned stock in Pfizer Inc during the time of this study and development of the manuscript, is currently an employee of CSL Behring Biotherapies for Life™. Melvin Toh, who was employed by and owned stock/options in Pfizer Inc at the time of this study, is currently an employee of CK Life Sciences Int’l. (Holdings) Inc and no longer owns stock/options in Pfizer Inc. Nenad Sarapa, who was employed by and owned stock/options in Pfizer Inc at the time of this study, is currently employed by Bayer Healthcare Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. A. Tortorici was employed at Pfizer Inc during the time of this study and development of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruiz-Garcia, A., Houk, B.E., Pithavala, Y.K. et al. Effect of axitinib on the QT interval in healthy volunteers. Cancer Chemother Pharmacol 75, 619–628 (2015). https://doi.org/10.1007/s00280-015-2677-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2677-z