Abstract
Purpose
Nonalcoholic steatohepatitis (NASH) has been associated with irinotecan (IRI)-based cancer chemotherapy regimens. The purpose of this study was to propose and test a consistent model of IRI-induced NASH, filling a gap in the medical literature.
Methods
Swiss male mice were distributed in groups (n = 8) and injected with saline (5 mL/kg, i.p.; control) or IRI (25, 50, 75 or 100 mg/kg, i.p.) thrice a week for 7 weeks. Blood samples were collected to measure the serum concentrations of proteins, alanine and aspartate aminotransferases (ALT and AST). Each week animals were euthanized, and the livers were submitted to myeloperoxidase (MPO) assay, lipid dosage, immunohistochemistry for inducible nitric oxide synthase (iNOS), TNF-α and interleukin-1β (IL-1β), and histopathological analysis. Survival rates were also determined.
Results
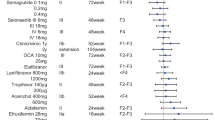
Mice treated with IRI had a significantly (p < 0.05) lower survival rate than controls and time- and dose-dependent body weight loss. ALT and AST plasma levels increased in relation to controls only in mice receiving IRI 50 mg/kg (p < 0.05). The histopathological features characteristic of NASH was observed, including steatosis, lobular neutrophil infiltration and ballooning hepatocytic degeneration. Additional findings included increased MPO, lipid accumulation, portal neutrophil infiltration, IL-1β and iNOS expression and fibrosis in liver tissues and low serum protein levels compared to controls.
Conclusion
This is the first report of a consistent model of IRI-induced NASH capable of mimicking clinical findings.




Similar content being viewed by others
References
Ludwig J, Viggiano TR, McGill DB, Oh BJ (1980) Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 55:434–438
Mulhall BP, Ong JP, Younossi ZM (2002) Non-alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol 17:1136–1143
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474
Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ (1999) Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116:1413–1419
Kleiner DE, Brunt EM, Natta MV, Behling C, Contos MJ, Cummings OW et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321
Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM et al (2006) Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24:2065–2072
Robinson SM, Wilson CH, Burt AD, Manas DM, White SA (2012) Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. doi:10.1245/s10434-012-2438-8
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343(13):904–914
House MG, Ito H, Gonnen M, Fong Y, Allen PJ, DeMatteo RP et al (2010) Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1600 patients during two decades at a single institution. J Am Coll Surg 210(5):744–752
Bismuth H, Adam R, Le′vi F, Farabos C, Waechter F, Castaing D et al (1996) Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 224:509–522
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D et al (2004) Rescue surgery for unresectable colorectal liver metastasis downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657
Zorzi D, Laurent A, Pawlick TM, Lauwers GY, Vauthey JN, Abdalla EK (2007) Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastasis. Br J Surg 94:274–286
Hammond JS, Guha IN, Beckingham IJ, Lobo DN (2011) Prediction, prevention and management of postresection liver failure. Br J Surg 98:1188–1200
Day CP, Jame OF (1998) Steatohepatitis: a tale of two ‘hits’? Gatroenterology 114:842–845
Melo ML, Brito GA, Soares RC, Carvalho SB, Silva JV, Soares PM et al (2008) Role of cytokines (TNF-α, IL-1β and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide. Cancer Chemother Pharmacol 61(5):775–784
Bradley PP, Christensen RD, Rothstein G (1982) Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60:618–622
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Hsu SM, Raine L (1981) Protein A, avidin and biotin in immunohistochemistry. J Histochem Cytochem 29:1349–1353
Fulco RA, Costa RC, Germano MP, Torre EA, Viscomi MG, Salimbeni V et al (2000) Hepatotoxicity of camptothecin derivatives in a primary culture system of rat hepatocytes. J Chemother 12(4):345–351
Keizman D, Maimon N, Ish-Shalom M, Buchbut D, Inbar M, Klein B et al (2010) An animal model for chemotherapy-associated steatohepatitis and its prevention by the oral administration of fatty acid bile acid conjugate. Cancer 116(1):251–255
Mikalauskas S, Mikalauskiene L, Bruns H, Nickkholgh A, Hoffmann K, Longerich T et al (2011) Dietary glycine protects from chemotherapy-induced hepatotoxicity. Amino Acids 40(4):1139–1150
Rasheed AZ, Rubin AH (2008) Toposiomerase-interactiona agents. In: DeVita VT, Lawrence TS, Rosenberg SA (eds) Devita, Hellman & Rosenberg’s cancer: principles & practice of oncology, 8th edn. Lippincott Williams & Wilkins, Philadelphia, pp 439–440
Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52:1836–1846
Dinarello CA (2000) Proinflammatory cytokines. Chest 118:503–508
Valles SL, Blanco AM, Azorin I, Guasch R, Pascual M, Gomez-Lechon MJ et al (2003) Chronic ethanol consumption enhances interleukin-1 mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Res 27(12):1979–1986
Garcia-Monzon C, Martin-Perez E, Iacono OL, Fernández-Bermejo M, Majano PL, Apolinario A et al (2000) Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol 33:716–724
Ha SK (2010) CHAE C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet induced obesity. Exp Anim 59(5):595–604
Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC et al (1993) Cytokines, endotoxin and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci 90:522–526
Uslusoy HS, Nak SG, Gulten M (2011) Noninvasive predictors for liver fibrosis in patients with nonalcoholic steatohepatitis. World J Hepatol 3(8):219–227
Kleiner DE, Brunt EM (2012) Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 32:2–13
Acknowledgments
We are grateful to Maria Silvandira Freire and Karina Felismino da Silva Santos for technical assistance. The authors received financial support from CNPq [National Council for Research and Development], CAPES [Brazilian government program for continuing higher education] and FUNCAP [Ceará State Foundation for Research Support]. RAR, MHLPS and GACB are CNPq scholarship holders.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, M.L.V., Lima-Júnior, R.C.P., Aragão, K.S. et al. Chemotherapy-associated steatohepatitis induced by irinotecan: a novel animal model. Cancer Chemother Pharmacol 74, 711–720 (2014). https://doi.org/10.1007/s00280-014-2434-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2434-8